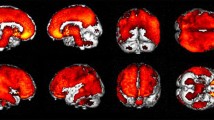
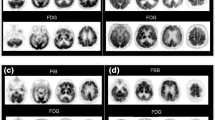
Abstract
Objective
We have encountered occasional equivocal findings when assessing cerebral cortical amyloid retention with 11C-Pittsburgh compound B (PiB) PET. We investigated the diagnostic significance of equivocal PiB PET findings.
Methods
This retrospective study included 101 consecutive patients complaining of cognitive disorders (30 Alzheimer’s disease, 25 mild cognitive impairment, 8 Lewy body disease, 7 frontotemporal lobar degeneration, 31 others) who underwent both 11C-PiB PET and 18F-fluorodeoxy-d-glucose (FDG) PET. We visually classified PiB-positive, PiB-equivocal or PiB-negative ratings according to cortical uptake. For quantitative assessments of PiB PET, standard uptake values referred to cerebellar cortex (SUVR) were calculated in regional template volume of interests (frontal, temporoparietal, precuneus/posterior cingulate cortex, cerebral white matter and cerebellar cortex). The results of visual assessment were compared with the regional and mean cortical SUVRs and cortical-to-white matter ratio of PiB uptake, as well as clinical and FDG PET findings.
Results
Among the 101 scans, 41 were PiB negative, 11 were PiB equivocal, and 49 were rated PiB positive in the visual assessments. The mean cortical SUVR and cortical-to-white matter ratio were 0.97 ± 0.07 and 0.57 ± 0.21 in PiB-negative, 1.51 ± 0.17 and 0.75 ± 0.06 in PiB equivocal and 2.10 ± 0.33 and 0.97 ± 0.11 in PiB-positive group, respectively. Nine of 11 subjects with PiB-equivocal findings had cognitive impairments and FDG distribution compatible with Alzheimer’s disease or dementia with Lewy bodies.
Conclusions
We considered equivocal visual findings on PiB PET equivalent to PiB-positive with slight cortical uptake. In addition, slight cortical amyloid deposits were considered to cause cerebral metabolic abnormality and cognitive impairment. Although mean cortical SUVR was more sensitive than visual assessment because of low cortical-to-white matter contrast due to non-specific accumulation in white matter, it is important not to overlook small amounts of cortical uptake of PiB in visual inspection for exact diagnosis.



Similar content being viewed by others
References
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburg compound-B. Ann Neurol. 2004;55:306–19.
Klunk WE. Amyloid imaging as a biomarker for cerebral β-amyloidosis and risk prediction for Alzheimer dementia. Neurobiol Aging. 2011;32:S20–36.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko S, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound B. J Cereb Blood Flow Metabol. 2005;25:1528–47.
Yaqub M, Tolboom N, Boellaard R, van Berckel BNM, van Triburg EW, Luurtsema G, et al. Simplified parametric methods for [11C] PIB studies. Neuroimage. 2008;42:76–86.
Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, et al. Simplified quantitation of Pittsburgh compound B amyloid imaging PET studies: a comparable analysis. J Nucl Med. 2005;46:1959–72.
Van Berckel BNM, Ossenkoppele R, Tolboom N, Yaqub M, Foster-Dingley JC, Windhorst AD, et al. Longitudinal amyloid imaging using 11C-PiB: methodological consideration. J Nucl Med. 2013;54:1570–6.
McKhann G, Drachman D, Foistein M, Katzman R, Price D, Stadian EM. Clinical diagnosis of Alzheimer’s disease-report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939.
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies. Third report of the DLB consortium. Neurology. 2005;65:1863–72.
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration—a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54.
Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–54.
Federo-Tavoletti MT, Rowe CC, McLean CA, Leone L, Li QX, Masters CL, et al. Characterization of PiB binding to white matter in Alzheimer disease and other dementias. J Nucl Med. 2009;50:198–204.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94.
Minoshima S, Foster NL, Petrie EC, Albin RL, Frey KA, Kuhl DE. Neuroimaging in dementia with Lewy bodies; metabolism, neurochemistry, and morphology. J Geriatr Psychiatry Neurol. 2002;15:200–9.
Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–45.
Ng S, Villemagne VL, Berlangieri S, Lee ST, Cherk M, Gong SJ, et al. Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. J Nucl Med. 2007;48:547–52.
Suotunen T, Hirvonen J, Immonen-Raiha P, Aalto S, Lisinen I, Arponen E, et al. Visual assessment of [11C]-PIB PET in patients with cognitive impairment. Eur J Nucl Med Mol Imaging. 2010;37:1141–7.
Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59:1152–60.
Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, et al. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–15.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Villemagne VL, Klunk WE, Mathis CA, Rowe CC, Brooks DJ, Hyman BT, et al. Aβ Imaging: feasible, pertinent, and vital to progress in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39:209–19.
Acknowledgments
We thank Mr. Yoshiyuki Nakayama for his support for brain FDG PET and PIB-PET at Kinki University Hospital. This study was supported in part by JSPS KAKENHI Grant Number 50534103 and the 21st Century Research and Development Incentive Wages at Kinki University.
Conflict of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosokawa, C., Ishii, K., Hyodo, T. et al. Investigation of 11C-PiB equivocal PET findings. Ann Nucl Med 29, 164–169 (2015). https://doi.org/10.1007/s12149-014-0924-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-014-0924-8