Abstract
Purpose
The purpose of our study was to evaluate the degree of radiotoxicity to lymphocytes in thyroid cancer after iodine-131(I-131) therapy using γ-H2AX foci immunodetection.
Methods
This study focused on 15 patients who underwent I-131 therapy for differentiated thyroid cancer after surgery. All patients received 3.7 GBq of I-131. Venous blood samples were collected from each patient before therapy and 4 days thereafter. Lymphocytes were isolated from the blood samples and subjected to γ-H2AX immunofluorescence staining.
Results
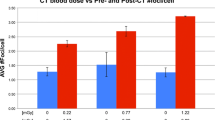
The number (mean ± SD) of foci per lymphocyte nucleus was 0.41 ± 0.51 before and 6.19 ± 1.80 after radioiodine therapy, and this difference was statistically significant (P = 0.001 < 0.05). Absorbed doses estimated for the 15 patients were 0.77 ± 0.31 Gy applying standard line in vitro external radiation doses.
Conclusion
γ-H2AX foci immunodetection in lymphocytes may detect radiation-induced DNA damage associated with I-131 therapy for thyroid cancer, and may facilitate estimation of the radiation doses absorbed with this therapy.


Similar content being viewed by others
References
Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJG, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–59.
Hurley JR, Becker DV. Treatment of thyroid carcinoma with radioiodine. In: Gottshalk A, Hoffer PB, Potchen EJ, editors. Diagnostic nuclear medicine. 2nd ed. Baltimore: Williams & Wilkins; 1988. p. 792–814.
Hurley JR, Becker DV. The use of radioiodine in the management of thyroid cancer. In: Freeman LM, Weissman HS, editors. Nuclear medicine annual. New York: Raven Press; 1983. p. 329–84.
Chow SM. Side effects of high-dose radioactive iodine for ablation or treatment of differentiated thyroid carcinoma. J HK Coll Radiol. 2005;8:127–35.
Leeper R. Controversies in the treatment of thyroid cancer. The New York Memorial Hospital approach. Thyroid Today 1982;5:1–6.
Maxon HR, Smith HS. Radioiodine-131 in the treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am. 1990;19:685–718.
M’Kacher R, Legal JD, Schlumberger M, Vosin P, Aubert B, Gaillard N, et al. Biological dosimetry in patients treated with iodine-131 for differentiated thyroid carcinoma. J Nucl Med. 1996;37:1860–4.
Monsieurs MA, Thierens HM, van de Wiele C, Vral AM, Meirlaen IA, de Winter HA, et al. Estimation of risk based on biological dosimetry for patients treated with radioiodine. Nucl Med Commun. 1999;20:911–7.
Watanabe N, Kanegane H, Kinuya S, Shuke N, Yokoyama K, Kato H, et al. The radiotoxicity of 131I therapy of thyroid cancer: assessment by micronucleus assay of B lymphocytes. J Nucl Med. 2004;45:608–11.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68.
Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–15.
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–7.
Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. PNAS. 2003;100:5057–506.
Rothkamm K, Balroop S, Shekhdar J, Fernie P, Goh V. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology. 2007;242:244–51.
Kuefner MA, Grudzenski S, Hamann J, Achenbach S, Lell M, Anders K, et al. Effect of CT scan protocols on X-ray-Induced DNA double-strand breaks in blood lymphocyte of patients undergoing coronary CT angiography. Eur Radiol. 2010;20:2917–24.
Beels L, Bacher K, de Wolf D, Werbrouck J, Thierens H. γ-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization are we underestimating radiation risks? Circulation. 2009;10:1903–9.
Horn S, Barnard S, Rothkamm K. Gamma-H2AX-based dose estimation for whole and partial body radiation exposure. PLoS One. 2011;6(9):e25113.
Sak A, Grehl S, Erichsen P, Engelhard M, Graraß, Levegrün S, et al. γ-H2AX foci formation in peripheral blood lymphocytes of tumor patients after focal radiotherapy to different sites of the body: dependence on the dose-distribution, irradiated site and time from start of treatment. Int J Radiat Biol. 2007;83:639–52.
Lassmann M, Hänscheid H, Gassen D, Biko J, Meineke V, Reiners C, et al. In vivo formation of γ-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2010;51:1318–25.
Thomas JP, Lautermann J, Liedert B, Seiler F, Thomale J. High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Mol Phamacol. 2006;70:23–9.
Collis SJ, Schwaninger JM, Natanbi AJ, Keller TW, Nelson WG, Dillehay LE, et al. Evasion of early cellular response mechanisms following low level radiation induced DNA damage. J Biol Chem. 2004;279:49624–32.
Hall EJ, Giaccia AJ. Radiobiology for the radiologist: molecular mechanism of DNA and chromosome damage and repair. 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Willkins; 2012. p. 12–34.
Acknowledgments
This study was supported in part by a Grant-in-Aid for scientific research from the Japan Society for the promotion of Science, Grant for Collaborative Research from Kanazawa Medical University (C2009-5,C2010-3), Grant for Project Research from High-Tech Research Center of Kanazawa Medical University (H2010-10, H2011-10), Assist KAKEN from Kanazawa Medical University and Hokkoku foundation for cancer research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doai, M., Watanabe, N., Takahashi, T. et al. Sensitive immunodetection of radiotoxicity after iodine-131 therapy for thyroid cancer using γ-H2AX foci of DNA damage in lymphocytes. Ann Nucl Med 27, 233–238 (2013). https://doi.org/10.1007/s12149-012-0678-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-012-0678-0