Abstract
During pre-therapy evaluation for yttrium-90 (Y-90) radioembolization, it is uncommon to find severe imaging discordance between hepatic angiography versus technetium-99m-macroaggregated albumin (Tc-99m-MAA) single photon emission computed tomography with integrated low-dose CT (SPECT/CT). The reasons for severe imaging discordance are unclear, and literature is scarce. We describe 3 patients with severe imaging discordance, whereby tumor angiographic contrast hypervascularity was markedly mismatched to the corresponding Tc-99m-MAA SPECT/CT, and its clinical impact. The incidence of severe imaging discordance at our institution was 4% (3 of 74 cases). We postulate that imaging discordance could be due to a combination of 3 factors: (1) different injection rates between soluble contrast molecules versus Tc-99m-MAA; (2) different arterial flow hemodynamics between soluble contrast molecules versus Tc-99m-MAA; (3) eccentric release position of Tc-99m-MAA due to microcatheter tip location, inadvertently selecting non-target microparticle trajectories. Tc-99m-MAA SPECT/CT more accurately represents hepatic microparticle biodistribution than soluble contrast hepatic angiography and should be a key criterion in patient selection for Y-90 radioembolization. Tc-99m-MAA SPECT/CT provides more information than planar scintigraphy to guide radiation planning and clinical decision making. Severe imaging discordance at pre-therapy evaluation is ominous and should be followed up by changes to the final vascular approach during Y-90 radioembolization.





Similar content being viewed by others
References
Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, et al. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007;10:12–29.
Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin Nucl Med. 2010;40:105–21.
Wang SC, Bester L, Burnes JP, Clouston JE, Hugh TJ, Little AF, et al. Clinical care and technical recommendations for yttrium-90 microsphere treatment of liver cancer. J Med Imaging Radiat Oncol. 2010;54:178–87.
Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC, Leung TW et al. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys. 2010 (Epub ahead of print).
Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64.
Riaz A, Lewandowski RJ, Kulik LM, Mulcahy MF, Sato KT, Ryu RK, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121–30.
Kennedy AS, Kleinstreuer C, Basciano CA, Dezarn WA. Computer modeling of yttrium-90-microsphere transport in the hepatic arterial tree to improve clinical outcomes. Int J Radiat Oncol Biol Phys. 2010;76:631–7.
Basciano CA, Kleinstreuer C, Kennedy AS, Dezarn WA, Childress E. Computer modeling of controlled microsphere release and targeting in a representative hepatic artery system. Ann Biomed Eng. 2010;38:1862–79.
Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–78.
Kaplan WD, D’Orsi CJ, Ensminger WD, Smith EH, Levin DC. Intra-arterial radionuclide infusion: a new technique to assess chemotherapy perfusion patterns. Cancer Treat Rep. 1978;62:699–703.
Rodari A, Bonfanti G, Garbagnati F, Marolda R, Milella M, Buraggi GL. Microsphere angiography in hepatic artery infusion for cancer. Eur J Nucl Med. 1981;6:473–6.
Bledin AG, Kantarjian HM, Kim EE, Wallace S, Chuang VP, Patt YZ, et al. 99mTc-labeled macroaggregated albumin in intrahepatic arterial chemotherapy. AJR Am J Roentgenol. 1982;139:711–5.
Gotti EW. Microsphere angiography of the liver. J Nucl Med. 1978;19:433–4.
Kaplan WD, Ensminger WD, Smith EH, D’Orsi CJ, Levin DC. Intra-arterial hepatic infusion of 99mTc-MAA: a predictive test of chemotherapeutic response of liver tumors. J Nucl Med. 1979;20:675.
Leung TW, Lau WY, Ho SK, Chan M, Leung NW, Lin J, et al. Determination of tumour vascularity using selective hepatic angiography as compared with intrahepatic-arterial technetium-99m macroaggregated albumin scan in hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33:S33–6.
Ho S, Lau WY, Leung TW, Chan M, Chan KW, Lee WY, et al. Tumor-to-normal uptake ratio of 90Y microspheres in hepatic cancer assessed with 99Tcm macroaggregated albumin. Br J Radiol. 1997;70:823–8.
Lau WY, Leung TW, Ho S, Chan M, Leung NW, Lin J, et al. Diagnostic pharmaco-scintigraphy with hepatic intra-arterial technetium-99m macroaggregated albumin in the determination of tumour to non-tumour uptake ratio in hepatocellular carcinoma. Br J Radiol. 1994;67:136–9.
Ho S, Lau WY, Leung TW, Chan M, Johnson PJ, Li AK. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med. 1997;24:293–8.
Campbell JM, Wong CO, Muzik O, Marples B, Joiner M, Burmeister J. Early dose response to yttrium-90 microsphere treatment of metastatic liver cancer by a patient-specific method using single photon emission computed tomography and positron emission tomography. Int J Radiat Oncol Biol Phys. 2009;74:313–20.
Flamen P, Vanderlinden B, Delatte P, Ghanem G, Ameye L, Van Den Eynde M, et al. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with Yttrium-90 labeled resin microspheres. Phys Med Biol. 2008;53:6591–603.
GE Healthcare. Omnipaque (iohexol) injection prescribing information. 2009.
Gulec SA, Mesoloras G, Dezarn WA, McNeillie P, Kennedy AS. Safety and efficacy of Y-90 microsphere treatment in patients with primary and metastatic liver cancer: the tumor selectivity of the treatment as a function of tumor to liver flow ratio. J Transl Med. 2007;5:15.
Mallinckrodt Medical BV, LE Petten, Holland. Technescan LyoMAA package insert. 28 June 2007.
Kleinstreuer C. Methods and devices for targeted injection of radioactive microspheres. US Patent Application 61/127,889, July 28,2009, NC State University, Raleigh.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12149_2011_516_MOESM2_ESM.tif
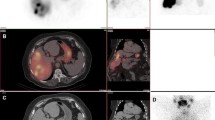
SUPPLEMENTAL FIGURE 1: Patient 1. Triphasic liver CT shows the segment VII hepatocellular carcinoma (HCC) in transaxial view (Fig. 1A arterial phase; Fig. 1B delayed phase). Fig. 1C shows two HCC masses in segments V/VI and VII in coronal view (TIFF 3446 kb)
12149_2011_516_MOESM3_ESM.tif
SUPPLEMENTAL FIGURE 2: Patient 2. Digital subtraction angiogram (DSA) obtained with microcatheter tip (C) in the replaced right hepatic artery demonstrates good contrast hypervascularity in the segment V HCC (Figs. 2A and 2B, arrows: tumor). Corresponding catheter-directed CT hepatic angiogram (CTHA) showed good arterial contrast enhancement in the segment V tumor (Fig. 2C). Tc-99m-MAA was slowly injected at this location. Tc-99m-MAA SPECT/CT showed marked tumoral photopenia in the arterial territory of the replaced right hepatic artery (Fig. 2D, arrow: tumor). Fig. 2E: Tumoral photopenia (arrow) is accentuated by increasing the SPECT threshold of Fig. 2D. Mesenteric Tc-99m-MAA activity in Fig. 2E is due to extra-hepatic shunting of injected Tc-99m-MAA into a branch of the left gastric artery, not relevant to the current context. The patient underwent Y-90 radioembolization with no change to the vascular approach. Post-radioembolization Y-90 time-of-flight PET/CT showed poor tumoral Y-90 activity in the arterial territory of the replaced right hepatic artery, concordant with Tc-99m-MAA SPECT/CT (Fig. 2F Y-90 PET/CT; Fig. 2G Y-90 PET; Fig. 2H non-contrast-enhanced CT component of PET/CT; arrows: tumor) (TIFF 3824 kb)
12149_2011_516_MOESM4_ESM.tif
SUPPLEMENTAL FIGURE 3: Patient 3. Triphasic liver CT in the arterial phase shows a single large HCC in the right lobe (Fig. 3A). The tumor was supplied by the right hepatic artery, which trifurcates into 3 tumoral branches: superior (S), middle (M) and inferior (I). DSA obtained with the microcatheter tip (C) proximal to its trifurcation demonstrated good contrast perfusion in all 3 tumoral branches (Fig. 3B). Tc-99m-MAA was slowly injected at this location. Tc-99m-MAA SPECT/CT showed preferential Tc-99m-MAA implantation in the arterial territory of the superior branch (S), while territories supplied by the middle (M) and inferior (I) branches were markedly photopenic, suggesting severe imaging discordance (Fig. 3C). The vascular approach for Y-90 radioembolization was changed in response to the Tc-99m-MAA SPECT/CT findings, and Y-90 resin microspheres were injected super-selectively with the microcatheter tip positioned into each of the 3 branches (Fig. 3D; actual super-selective DSA images not shown). Super-selective catheter-directed CTHA obtained at each of the 3 branches delineate the perfused arterial territories (Fig. 3E superior branch; Fig. 3F middle branch; Fig. 3G inferior branch). Post-radioembolization Y-90 time-of-flight PET/CT showed satisfactory tumoral microsphere implantation in all 3 arterial territories (Fig. 3H Y-90 PET/CT; Fig. 3I Y-90 PET). (TIFF 4632 kb)
Rights and permissions
About this article
Cite this article
Kao, Y.H., Tan, E.H., Teo, T.K.B. et al. Imaging discordance between hepatic angiography versus Tc-99m-MAA SPECT/CT: a case series, technical discussion and clinical implications. Ann Nucl Med 25, 669–676 (2011). https://doi.org/10.1007/s12149-011-0516-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-011-0516-9