Abstract
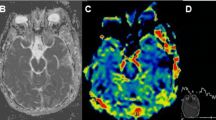
Assessment via MRI is an integral part of the management of primary brain tumors. However, reliance on imaging to determine treatment response is not without its pitfalls. Necrosis is a known late effect of radiation treatment of the brain that can mimic tumor recurrence. It is now appreciated that pseudoprogression, a similar effect, can occur after combined chemoradiotherapy and can occur more quickly and dramatically than after radiation alone. Although several adjunct imaging modalities are under investigation, none is yet widely accepted as being able to distinguish between true progression and pseudoprogression. Conversely, at disease progression, antiangiogenic therapies are frequently used and can have a rapid positive effect on imaging. These changes, increasingly known as “pseudoresponses,” can occur immediately after initiating treatment, making accurate assessment of true tumor response difficult. This article reviews the challenges of brain tumor imaging and its use in assessment of treatment response.
Similar content being viewed by others
References and Recommended Reading
Stupp RR, Mason WP, van den Bent MJ, et al.: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005, 352:987–996.
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990, 8:1277–1280.
Finn MA, Blumenthal DT, Salzman KL, Jensen RL: Transient postictal MRI changes in patients with brain tumors may mimic disease progression. Surg Neurol 2007, 67:246–250.
Ulmer S, Braga TA, Barker FG II, et al.: Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 2006, 67:1668–1670.
Chamberlain M, Glantz M, Chalmers L, et al.: Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 2007, 82:81–83.
Taal W, Brandsma D, de Bruin HG, et al.: Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008, 113:405–410.
Brandes AA, Franceschi E, Tosoni A, et al.: MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 2008, 26:2192–2197.
Hegi ME, Diserens AC, Gorlia T, et al: MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005, 352:997–1003.
Prados MD, Chang SM, Butowski N, et al.: Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol 2009, 27:579–584.
Mullins ME, Barest GD, Schaefer PW, et al.: Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 2005, 26:1967–1972.
Kumar AJ, Leeds NE, Fuller GN, et al.: Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000, 217:377–384.
Hein PA, Eskey CJ, Dunn JF, Hug EB: Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 2004, 25:201–209.
Hu LS, Baxter LC, Smith KA, et al.: Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 2009, 30:552–558.
Sugahara T, Korogi Y, Tomiguchi S, et al.: Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 2000, 21:901–909.
Shimizu H, Kumabe T, Tominaga T, et al.: Noninvasive evaluation of malignancy of brain tumors with proton MR spectroscopy. AJNR Am J Neuroradiol 1996, 17:737–747.
Vigneron D, Bollen A, McDermott M, et al.: Threedimensional magnetic resonance spectroscopic imaging of histologically confirmed brain tumors. Magn Reson Imaging 2001, 19:89–101.
Chen W: Clinical applications of PET in brain tumors. J Nucl Med 2007, 48:1468–1481.
Ricci P, Karis J, Heiserman J, et al.: Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 1998, 19:407–413.
Terakawa Y, Tsuyuguchi N, Iwai Y, et al.: Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 2008, 49:694–699.
Chen W, Cloughesy T, Kamdar N, et al.: Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18FFDG. J Nucl Med 2005, 46:945–952.
Xiangsong Z, Weian C: Differentiation of recurrent astrocytoma from radiation necrosis: a pilot study with 13N-NH3 PET. J Neurooncol 2007, 82:305–311.
Vredenburgh JJ, Desjardins A, Herndon JE II, et al.: Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007, 13:1253–1259.
Vredenburgh JJ, Desjardins A, Herndon JE II, et al.: Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007, 25:4722–4729.
Cloughesy TF, Prados MD, Wen PY, et al.: A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) [abstract 2010b]. J Clin Oncol 2008, 26(Suppl).
Pope WB, Lai A, Nghiemphu P, et al.: MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 2006, 66:1258–1260.
Batchelor TT, Sorensen AG, di Tomaso E, et al.: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007, 11:83–95.
Norden AD, Young GS, Setayesh K, et al.: Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008, 70:779–787.
Raymond E, Brandes AA, Dittrich C, et al.: Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol 2008, 26:4659–4665.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clarke, J.L., Chang, S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr Neurol Neurosci Rep 9, 241–246 (2009). https://doi.org/10.1007/s11910-009-0035-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-009-0035-4