Abstract
Cardiac involvement portends a poor prognosis in patients with sarcoidosis. However, due to the nonspecific clinical manifestations of the disease, patchy myocardial involvement, and the limited diagnostic yield of diagnostic tests, early diagnosis of cardiac sarcoidosis has been exceedingly difficult. As a result, there is no standardized approach for the early diagnosis of cardiac sarcoidosis. Imaging modalities that can both identify disease and predict response to therapy are paramount to improve management of cardiac sarcoidosis. 18F-FDG PET has many practical advantages in assessing disease activity and monitoring treatment response in patients with cardiac sarcoidosis. Accumulating data support the growing role of 18F-fluorodeoxyglucose (18F-FDG) PET in the diagnosis and risk stratification of patients with cardiac sarcoidosis.
Similar content being viewed by others
Introduction
In the United States, cardiac sarcoidosis (CS) represents the cause of death in as many as 13 % to 25 % of fatal cases of sarcoidosis [1]. Although cardiac involvement portends a higher risk of mortality and morbidity [2], ante-mortem diagnosis of CS remains challenging based on a broad and nonspecific set of signs and symptoms ranging from asymptomatic electrocardiographic findings to sudden death and progressively worsening heart failure and arrhythmias [3, 4, 5•, 6••]. Indeed, approximately 25 % of all patients with sarcoidosis have clinical manifestations of cardiac involvement whereas myocardial granulomas can be identified in almost 25 %–79 % of autopsy examinations [7]. Traditionally, the Japanese Ministry of Health and Welfare Guidelines (Japanese Ministry Guidelines), published 20 years ago [8], and revised more recently [9••, 10], have attempted to provide diagnosis criteria that yield a “histologic diagnosis” or “clinical diagnosis” (Table 1). A positive endomyocardial biopsy can clinch the histologic diagnosis of CS. However, this procedure carries risks and lacks sensitivity (19 % sensitivity) [11, 12] given the heterogeneous myocardial involvement [4, 13]. Recently, imaging with positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) has gained momentum in the management of patients with CS [14, 15]. In this review we detail the technical aspects of 18F-FDG PET imaging and discuss its role in the clinical diagnosis, assessment of disease activity, monitoring therapeutic response, and assessment of prognosis for myocardial sarcoidosis.
Pathophysiology and Presentation
The basic pathophysiological abnormality in sarcoidosis is the accumulation of noncaseating granulomas comprised of organized collections of macrophages, epithelioid cells, and lymphocytes [16••]. These granulomas may initially form to encircle the inciting pathogen such as infectious (mycobacterium or corynebacterium species) or environmental agents (aluminum, talk, pollen, moldy environments, insecticides, inorganic particles) to protect the surrounding tissue from damage [16••, 17]. In the process, CD4+ T-cells are activated leading to inflammation through release of interferon-γ (INF-γ) or interleukin-2 (IL-2). Tissue scarring may result through a shift in cytokine production from interleukin-2 (IL-2) and interferon-γ (INF-γ) to interleukins 4, 10, and 13 (IL-4, 10, 13) [16••].
In the heart the granulomas may involve the pericardium, myocardium, and endocardium of both the ventricles as well as the atria [17]. The myocardium of the left ventricular free wall, particularly at the base of the heart, is most commonly affected, followed by the basal interventricular septum. Also, there appears to be a predilection for involvement of the conduction system.
Cardiac sarcoidosis clinical manifestations relate primarily to the location and inflammatory effects of the granulomas [16••]. The presence of the granulomas in the ventricular myocardium may lead to abnormal automaticity and re-entrant tachyarrhythmias manifesting as palpitations or syncope. Involvement of the conduction system may lead to bradyarrhythmias and syncope. Congestive heart failure may result from widespread sarcoidosis of the myocardium with a decline in left ventricular ejection fraction (EF) and death. Unlike with other infiltrative cardiomyopathies such as amyloidosis, myocardial involvement in sarcoidosis is typically patchy. This may account for the low yield from right ventricular endomyocardial biopsy, which is typically a blind procedure with sampling of the right ventricular aspect of the interventricular septum. Due to the challenges in histological diagnosis from endomyocardial biopsy, imaging plays a critical role in the diagnosis of CS.
Imaging of Myocardial Inflammation
Several radionuclide techniques are available to image inflammation [18] using 18F-FDG, 99mTechnetium (99mTc), or 111Indium (111In) labeled white blood cells, 99mTc bisphosphonates, 67Gallium-citrate, 99mTc nanocolloids, and 99mTc or 111In labeled proteins (IgG, albumin). 67Gallium-citrate imaging has low sensitivity and limited spatial resolution for the detection of sarcoidosis in the extrapulmonary locations, compared with 18F-FDG PET [19]. Hence, 18F-FDG PET imaging is most commonly used for imaging myocardial inflammation in clinical practice. The cellular uptake of 18F-FDG in sarcoidosis is related to these inflammatory cell infiltrates [20–22]. Active inflammatory cells have high glycolytic activity to satisfy their large energy demands [23], and as a result can produce ATP levels that are almost 8-fold higher than baseline [24]. After crossing the cellular membrane via glucose transporters, both 18F-FDG and glucose are phosphorylated by the enzyme hexokinase. While glucose is further metabolized, the phosphorylated 18F-FDG remains trapped within the cells and can be imaged [25, 26]. As 18F-FDG accumulates in active lesions where inflammatory cells utilize glucose as an energy source, active sarcoid lesions in various organs are visualized by increased 18F-FDG uptake on PET imaging.
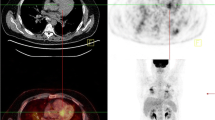
Typical radionuclide protocols for imaging cardiac sarcoidosis include 18F-FDG PET for imaging inflammation combined with SPECT or PET myocardial perfusion imaging (Fig. 1).
Perfusion and 18F-FDG PET imaging protocol. All patients are instructed to take a high-fat low-carbohydrate diet the day prior with or without additional overnight fast. A rest myocardial perfusion imaging study is first performed followed by injection of 18F-FDG. Some sites use IV (intravenous) unfractionated heparin (50 units/kg) about 15 minutes prior to the IV injection of 10–12 mCi of 18F-FDG. After a 90-minute uptake period, cardiac and partial whole body PET images (cerebellum to mid-thigh) are acquired
18F-FDG PET: Technical Details
Patient Preparation
Since normal myocytes utilize glucose as one of their main energy sources, various techniques have been developed to suppress physiological 18F-FDG uptake in the heart and minimize false-positive results. In this regard, 3 approaches have been tried: prolonged fasting, dietary modification, and intravenous administration of unfractionated heparin.
In the fasting state, free fatty acids account for up to 90 % of oxygen consumption of normal myocytes. However, in the post-prandial state, when plasma glucose and insulin levels are increased, glucose can actually become the dominant source of energy. As a result, fasting imaging is preferable over post-prandial imaging, in order to decrease physiologic uptake by normal myocytes and optimize 18F-FDG PET imaging [14, 20, 21]. While many studies have been conducted with 5–12 hours of fasting, more recent studies suggest that waiting more than 18 hours further suppresses physiologic myocardial glucose uptake and may be preferable [27, 28]. However, fasting imaging remains limited by variable suppression of glucose utilization by normal myocytes.
Dietary modifications with a high-fat, high-protein, low-carbohydrate diet have been shown to reduce glucose uptake by normal myocardium [29••, 30]. More recently, low-carbohydrate diet followed by extended fasting have been studied, and shown to achieve significant reduction in physiologic uptake [31]. The idea is to shift myocardial metabolism to fatty acid and suppress glucose utilization and 18F-FDG uptake by the normal myocardium.
Finally, some protocols utilize pre-administration of unfractionated intravenous (IV) heparin for suppressing myocardial 18F-FDG uptake. IV unfractionated heparin activates lipoprotein and hepatic lipases, thereby increasing plasma free fatty acid levels, and ultimately causing a reduction in glucose consumption of normal myocytes. Doses as high as 50 IU/Kg were used in prior studies [19, 20] without describing any bleeding complications; it remains unclear whether lower heparin dosing could result in optimal quality imaging [20].
Although not formally tested, it is possible that a combination of all of the above techniques, ie, high-fat low carbohydrate diet for 2 meals followed by prolonged fasting as well as the use of intravenous unfractionated heparin may be superior to either one technique alone.
18F-FDG Imaging Protocol
Following the dietary preparation mentioned above, patients receive 10–12 milli Curies of 18F-FDG intravenously. 18F-FDG PET imaging is begun after an uptake period of 90 minutes during which patients are asked to rest quietly (not talk or engage in physical activity) (Fig. 1). The heart position is localized with a scout image (CT or radionuclide) and a transmission scan (CT or radionuclide) is obtained for the measurement and correction of soft tissue attenuation followed by a non-gated cardiac 18F-FDG image acquisition (2D or 3D mode). Then, a limited whole body scan is obtained from the base of the skull to mid-thigh along with a transmission scan for attenuation correction. The cardiac and whole body images emission images are reconstructed with and without attenuation correction.
Myocardial Perfusion Imaging Protocol
All patients scheduled for 18F-FDG PET for cardiac sarcoidosis should also undergo myocardial perfusion assessment with 99m Tc, 201Thallium,13N-ammonia, or 82Rubidium based radiotracers using standard protocols [32, 33]. Attenuation correction should be used with SPECT myocardial perfusion imaging whenever possible. We recommend perfusion assessment both at the initial diagnosis and for the follow-up evaluations to assess response to therapy. Comparison of the perfusion images with 18F-FDG PET images will allow for differentiation of scar tissue from normal myocardium or change of active sarcoid granuloma to normal myocardium or progression to scar tissue in response to therapy. It is also important to exclude epicardial coronary artery disease in these patients with either invasive or CT based coronary angiography, since patterns of perfusion metabolism mismatch in patients with left ventricular systolic dysfunction may reflect either hibernating myocardium or myocardial inflammation based on the clinical context.
Interpretation of the Perfusion and 18F-FDG PET Images
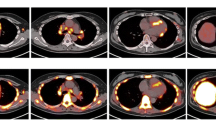
The perfusion and 18F-FDG PET images are interpreted side-by-side using conventional cardiac display software and a semi-quantitative visual interpretation. Several descriptions have been used for 18F-FDG uptake, but, not yet validated with histological disease stage. One description on the 18F-FDG PET images is: no uptake, diffuse uptake, focal uptake, and focal on diffuse uptake [9••]. Other investigators have used patterns incorporating information from the perfusion and information from the 18F-FDG PET images: both normal perfusion and normal 18F-FDG, either abnormal perfusion or abnormal 18F-FDG, or both abnormal perfusion and abnormal 18F-FDG [34]. Yet another way to describe the patterns is based on a comparison of the degree of perfusion abnormality and 18F-FDG uptake as: normal (normal perfusion/normal 18F-FDG), early stage (mild perfusion defect/increased 18F-FDG), progressive stage (moderate perfusion defect/increased 18F-FDG), progressive myocardial impairment stage (severe perfusion defect/increased 18F-FDG), and fibrosis stage (severe perfusion defect/minimal or no 18F-FDG uptake) [21]. These patterns are summarized in Fig. 2. These stages can be helpful not only in the initial diagnosis, but, also in follow-up of patients who return for assessment of response to therapy.
For assessment of response to therapy several investigators have relied on the specific uptake values (SUV) of 18F-FDG to monitor response to therapy [21]. Specific uptake values represent the decay corrected uptake in the tissue divided by the injected dose of radiotracer adjusted to body weight [35]. The ratio of SUV values in the myocardium to the SUV values in other organs, such as cerebellum, has also been used. Others have relied on the measure of coefficient of variation of the SUV value as a metric to assess response to therapy [9••]. The clinical value of these SUV metrics to assess disease activity level at baseline and to assess changes in response to therapy is not well established and needs to standardized and prospectively validated.
The whole body 18F-FDG PET as well as the CT transmission (on hybrid scanners) images need to be reviewed and reported in conjunction with a physician credentialed to supervise and interpret body PET/CT. Findings supporting a diagnosis of extra-cardiac sarcoid disease activity or findings to guide a biopsy from an extracardiac location of 18F-FDG uptake (18F-FDG avid lymph nodes accessible to guided biopsy) as well as incidental diagnosis of malignancy should be considered.
Role of 18F-FDG PET in the Initial Diagnosis of Myocardial Sarcoidosis
The early diagnosis of cardiac sarcoidosis is imperative, as data suggest that high dose steroid therapy should be administered to patients with active inflammation before left ventricular systolic function declines [3, 36, 37]. The traditional approach of reserving steroids for patients with LVEF <50 %, advanced atrioventricular block, ventricular tachycardia, or positive cardiac biopsy led to high mortality risk [38, 39]. It is therefore suspected that there is a tipping point of steroid efficacy where responsive active granulomatous inflammation/infiltration transforms toward non-responsive fibrosis [40••].
Several recent studies have demonstrated the promising potential of using PET with 18F-FDG in the early diagnosis and assessment of cardiac sarcoidosis (Table 2). Yamagishi et al. [14] enrolled 17 patients with pathology proven systemic sarcoidosis and cardiac involvement based on the Japanese Ministry Guidelines, and compared 13N-ammonia/18F-FDG PET imaging vs 67Gallium and 201Thallium scintigraphy. They showed that 18F-FDG accumulates in patients with cardiac sarcoidosis, similar to what has been observed in patients with pulmonary involvement. Fifteen patients demonstrated increased myocardial 18F-FDG uptake, whereas only 6 patients demonstrated defects on 201Thallium scintigraphy, and only 3 patients showed 67Gallium uptake. Other investigators have similarly demonstrated higher sensitivity of 18F-FDG PET imaging compared with 67Gallium or 99mTechnetium sestamibi scintigraphy [20, 27]. Some even suggested that 18F-FDG PET can detect early stages of cardiac sarcoidosis where fewer perfusion abnormalities and less inflammatory activity are noted [21].
In one of the first studies comparing 18F-FDG PET with cardiac magnetic resonance imaging (CMR) [41] for the diagnosis of cardiac sarcoid, 21 patients with suspected cardiac sarcoidosis underwent both CMR and 18F-FDG within 4 weeks. Using the Japanese Ministry Criteria as the gold standard, 8 of 21 patients were diagnosed with cardiac sarcoidosis yielding a sensitivity and specificity for 18F-FDG PET of 87.5 % and 38.5 %, respectively, compared with 75 % and 76.9 % for CMR. A second study by Mehta et al. [23] similarly showed higher sensitivity for 18F-FDG PET (86 %) than for CMR (36 %) in patients with suspected cardiac sarcoid.
Many studies have shown that 18F-FDG has a characteristic heterogeneous uptake pattern in the myocardium in patients with cardiac sarcoidosis [14, 20, 21]. This appearance, as opposed to the diffuse uptake seen in dilated cardiomyopathy and normal subjects, should theoretically help distinguish patients with cardiac sarcoidosis and decrease false positive rates. Tahara et al. [9••] compared the quantitative and qualitative features of 18F-FDG uptake in sarcoidosis patients with and without cardiac involvement, in addition to patients with dilated cardiomyopathy and control subjects with normal hearts. They measured SUV values in the 17 myocardial segments and calculated the average SUV value and standard deviation of the SUV value. Then a coefficient of variation (as a metric of image heterogeneity) was computed as the SD of SUV divided by the average of SUV. The results of that study demonstrated that 18F-FDG uptake was distinctly heterogeneous in patients with cardiac sarcoidosis with a higher coefficient of variation of the SUV (0.25 ± 0.05) compared with control subjects (0.14 ± 0.03, P < 0.01) and compared with subjects with non cardiac sarcoidosis (0.14 ± 0.03, P < 0.01) and dilated cardiomyopathy (0.15 ± 0.02, P < 0.01), and therefore diagnostic of the disease.
While 18F-FDG PET has not been included in the most recent update to the Japanese Ministry guidelines [9••, 10], the existing data to date support the role of 18F-FDG PET as a sensitive technique for the diagnosis of cardiac sarcoidosis.
Role of 18F-FDG PET in the Management of Patients with Cardiac Sarcoidosis
Although not prospectively tested in double-blinded randomized clinical trials, early administration of corticosteroid therapy in patients with revealed active cardiac sarcoidosis has been associated with prevention of left ventricular function decline and remodeling [3, 36, 37]. Based on several retrospective small cohort analyses, it is suggested to start oral prednisone at 30 mg daily [16••] (or 60 mg every other day) for 3 months and eventually initiate a long tapering process over several months with serial monitoring of disease activity with imaging and dose adjustments to decide on minimal maintenance dose or complete withdrawal. As suggested, cardiac imaging plays a relatively important role in this decision process (Fig. 3), however the evidence supporting this approach is not without controversy.
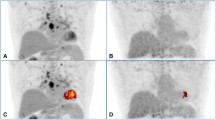
PET perfusion and 18F-FDG imaging at baseline and after 6 months of high-dose steroid therapy. These images were obtained in a 51-year-old man being evaluated for heart block and dizziness. A cardiac MRI was suggestive of cardiac sarcoidosis. Invasive coronary angiogram revealed normal epicardial coronary arteries. a, Baseline 82rubidium perfusion and 18F-FDG images in color in alternate rows displayed as short axis, horizontal long axis, and vertical long axis images along with partial whole body FDG images in grey scale in the coronal, sagittal, and transaxial projections. On the baseline images, there are small and mild, patchy perfusion defects in the basal and mid anteroseptal and inferoseptal walls with a corresponding mismatch on the FDG images. Increased 18F-FDG uptake is also noted in the right ventricular free wall. Regions of the myocardium with normal perfusion (lateral and inferior walls) show no 18F-FDG uptake consistent with excellent suppression of myocardial glucose utilization by the normal myocardium. The partial whole body images in grey scale show increased 18F-FDG uptake in the bilateral upper para-tracheal, anterior mediastinal, right lower para-tracheal, subcarinal, para-esophageal, and bilateral hilar lymph nodes. b, Post-treatment (6 months after high dose steroid therapy) 82rubidium perfusion and 18F-FDG images in color along with partial whole body FDG images in grey scale as described previously. The small and patchy perfusion defects in the anterior wall and the inferoseptal wall are improved. There was no myocardial 18F-FDG uptake (only blood pool activity) consistent with excellent suppression of myocardial glucose utilization by the normal myocardium along with no 18F-FDG uptake in the regions with previously increased 18F-FDG uptake, suggesting interval improvement with high dose steroid therapy. Improvement/near complete resolution of 18F-FDG uptake is noted in the heart and lymph nodes on the partial whole body images. The post- treatment images show more pronounced foci of splenic 1F-FDG uptake compared with baseline
In a stratified analysis by Chiu et al. [38] based on baseline left ventricular ejection fraction, only subjects with an EF of 30 %–55 % had a statistical improvement in ventricular function compared with patients with an EF <30 %. This finding and others similar contributed to suggest that there might be a threshold of left ventricular EF where recovery becomes unlikely despite immunosuppressive therapy, stressing the importance of early recognition and management of cardiac sarcoidosis [39, 40••, 42].
The role of 18F-FDG PET imaging in managing patients with cardiac sarcoidosis remains to be prospectively defined and supported by evidence. However, based on pathophysiologic and molecular imaging mechanisms, one may surmise scenari where it may be important. Specifically, to determine not only structural and pathologic changes due to cardiac sarcoidosis but also to assess disease activity and serial temporal changes potential changes that may be secondary to therapeutic interventions.
Yamagishi et al. [14] described 7 patients diagnosed with cardiac sarcoidosis based on the Japanese Ministry Guidelines criteria that were treated with corticosteroids and underwent a cardiac PET a month later. 18F-FDG uptake was markedly diminished in size and intensity in 5 patients and disappeared completely in 2 patients. Furthermore, 3 of these patients demonstrated improvements and/or disappearance of conduction abnormalities or ventricular tachycardias. In the study by Tahara et al. [9••], comparing patients with and without cardiac involvement of sarcoid, in addition to patients with dilated cardiomyopathy and control subjects with normal hearts, the coefficient of variation of the SUV decreased significantly in the 9 cardiac sarcoidosis patients treated with steroids (0.24 ± 0.05 to 0.14 ± 0.06, P < 0.005) to a level similar to that of dilated cardiomyopathy or healthy controls suggesting that this measure could be a surrogate of disease activity and potentially aid in following response to therapy.
A case-report also reported resolution of a complete heart block by ECG after 1 month of steroid therapy in parallel to disappearance of increased myocardial metabolic activity as evidence by 18F-FDG uptake on serial PET studies [15]. In another case report, Matthews et al. [43••] describe a patient presenting with congestive heart failure, biopsy-proven sarcoidosis, and imaging findings consistent with cardiac involvement as evidenced with extensive 18F-FDG uptake in both the left and right ventricles as well as multiple focal areas of myocardial hyperintense signals on T2-weighted MRI images and corresponding areas of late gadolinium enhancement. The authors go on to describe significant clinical improvement after initiation of optimal medical heart failure therapy and corticosteroids. Subsequent follow-up CMR imaging at 3- and 9 months demonstrated improvements in left ventricular EF as well as near disappearance of the abnormal T2 signals but persistent late gadolinium enhancement. However, the 3- and 12-month 18F-FDG PET demonstrated complete resolution of any cardiac, or extracardiac sarcoid activity, illustrating that while CMR and 18F-FDG PET were both helpful in establishing the diagnosis of cardiac sarcoid involvement, only 18F-FDG PET imaging serial imaging demonstrated serial findings mirroring disease activity correlating with potential response to therapy.
Role of 18F-FDG PET in Assessing Prognosis
Several studies have confirmed that cardiac involvement in sarcoidosis portends a poor prognosis [44]. Granulomatous infiltration can lead to progressively worsening heart failure [23] and life-threatening cardiac arrhythmias [23]. Sudden death was shown to be the most frequent cause of death among patients with cardiac sarcoidosis [11].
In an attempt at investigating whether subtle cardiac involvement on sensitive radiographic imaging modalities predicts severity of cardiac dysfunction and worse prognosis, Betensky et al. [45] retrospectively studied 45 patients with implantable cardiac defibrillators (ICDs) and biopsy-proven or highly suspected cardiac sarcoidosis based on advanced cardiac imaging. Twenty-six patients (58 %) had findings consistent with cardiac sarcoidosis on 18F-FDG PET imaging. These authors found that ventricular tachycardia (VT) and/or ventricular fibrillation (VF) requiring ICD therapies was common and significantly associated with longer device follow-up time, depressed left ventricular systolic function, and the presence of complete heart block. Interestingly, 4 of the 7 patients with preserved left ventricular EF and appropriate ICD therapy had positive PET scans (as opposed to positive CMR only), and the authors suggested that active myocardial inflammation may be a potential marker for VT risk in this subgroup. Furthermore, 11 of the 26 patients (42.3 %) with positive 18F-FDG scans had VT/VF as opposed to 2 of the 7 patients (28.6 %) with negative 18F-FDG scans and positive CMR or biopsy only. The authors concluded that in the setting of a positive 18F-FDG scan, an EF ≥ 35 % did not appear to be entirely protective with regard to arrhythmias.
Isiguzo et al. [25] conducted a retrospective chart review of 18 patients in order to ascertain the utility of 82Rb/18F-FDG PET scan for assessment of disease activity in patients with cardiac sarcoidosis. The positive predictive value of 82Rb and 18F-FDG PET scan showing over 6 % perfusion-metabolism mismatch for detecting clinically active cardiac sarcoidosis was 100 %. However, the test had lower specificity (62.5 %).The authors concluded that the finding of a greater than 6 % mismatch is highly indicative of clinically active disease, but more studies are required to predict prognosis.
Mehta et al. [23] prospectively evaluated 62 patients with biopsy-proven extracardiac sarcoidosis but with preserved left ventricular EF and without clinical evidence of cardiac involvement. Twenty-five of them were screened by 18F-FDG PET for cardiac involvement, and 22 of these patients (88 %) were found to have abnormalities and were referred for electrophysiologic programmed electrical stimulation (EPS) in order to determine their risk of sustained ventricular arrhythmias and sudden cardiac death. Over a mean follow-up of 1.8 years, no patient died or had ventricular arrhythmias, and the authors concluded that sarcoid lesions seen on 18F-FDG scans do not predict arrhythmias in patients with preserved cardiac function. However, it should be noted that the cohort studied excluded patients with pre-existing heart disease, which is a known predictor of survival in cardiac sarcoidosis [39]. Furthermore, several patients were on therapy with systemic corticosteroids, which may favorably modify the myocardial substrate.
In the largest study to date, Blankstein et al. [34] studied 125 patients who underwent myocardial perfusion imaging and 18F-FDG PET imaging following a low carbohydrate-high fat diet. These patients were followed up for 1.6 years for major cardiac events (n = 38) of ventricular tachycardia (n = 33) or death (n = 10). Subjects in this study were grouped according to perfusion/18F-FDG uptake: normal perfusion/normal 18F-FDG; either abnormal perfusion or abnormal 18F-FDG; or both abnormal perfusion and abnormal 18F-FDG. The authors showed that the presence of both a perfusion defect and abnormal 18F-FDG is associated with an increased risk of major cardiac events (hazard ratio of 2.8, P < .01) after adjusting for EF.
Further prospective studies with larger patient cohorts are needed to elucidate the diagnostic and prognostic relevance of 18F-FDG PET in the management of cardiac sarcoidosis.
Challenges with 18F-FDG Imaging for Myocardial Sarcoidosis
18F-FDG imaging is a sensitive test for the diagnosis of cardiac sarcoidosis. However, it is not widely available and is expensive. Techniques to suppress myocardial glucose utilization are not perfect. Patient instructions are crucial to ensure they follow the appropriate diet and despite dietary compliance, inadequate suppression of 18F-FDG uptake may be seen reducing test specificity [35]. Also, although 18F-FDG imaging has been used to assess response to therapy, the metabolic alterations related to prolonged corticosteroid therapy make it challenging to perform and interpret 18F-FDG images in these patients. Elevations in serum glucose and insulin levels due to steroids use may adversely affect 18F-FDG uptake by normal myocytes and reduce test specificity. Another challenge is the interpretation of decreased 18F-FDG uptake on the follow-up scan. Firstly, it may be hard to visualize a change on relative perfusion images (since the image normalizes to any 18F-FDG uptake). Hence, SUV values and comparison with reference organ uptakes are critical for appropriate interpretation of these images. Also, a reduction in myocardial 18F-FDG uptake could mean resolution of inflammation (normal myocytes) or progression of disease process and fibrosis. Perfusion imaging is therefore critical even on the follow-up scans to distinguish these 2 entities (see Fig. 2, panels normal and fibrosis), as perfusion should be normalized in the former and should be worse in the latter scenario.
Role of 18F-FDG PET in Assessing Cardiac Sarcoidosis in the era of Multimodality Cardiovascular Imaging
Several techniques can be used to image cardiac sarcoidosis. Echocardiography is easy to obtain and important for determination of left ventricular systolic function. Small focal wall motion abnormalities [46] or aneurysms in a non-coronary distribution and elevated pulmonary artery systolic pressure can be clues to sarcoid cardiac involvement. However, it is not easy to distinguish wall motion abnormalities related to cardiac sarcoidosis from coronary artery disease or other etiologies. Likewise, perfusion defects on myocardial perfusion imaging can be nonspecific. Also, perfusion imaging and 67Gallium imaging are relatively insensitive compared with 18F-FDG PET and gadolinium enhanced delayed CMR. Presently, 18F-FDG PET and gadolinium enhanced delayed CMR remain the main stays for the diagnosis of cardiac sarcoidosis.
Accumulating data support the role of 18F-FDG PET as an accurate technique likely to aid in the diagnosis of cardiac sarcoidosis. 18F-FDG PET has the advantage of high diagnostic accuracy and has been shown to be equally or more sensitive than delayed enhancement CMR in detecting myocardial lesions from cardiac sarcoidosis. Furthermore, 18F-FDG PET has several practical advantages over CMR as it and can be utilized in patients in whom CMR is contraindicated, as in patients with impaired renal function and/or those with implanted metallic devices. The percentage of patients with indwelling cardiac devices is not negligible in this population, as many patients with cardiac sarcoidosis may present with heart block requiring pacemaker or recurrent ventricular arrhythmias and ICD implants. 18F-FDG PET also offers assessment of disease activity based on SUV values as well as assessment of perfusion patterns, whereas CMR is somewhat limited in this capability [43••]. Furthermore, the acquisition of whole-body 18F-FDG PET/CT images has the advantage of evaluating the extent of systemic disease beyond the myocardium. Therefore, inclusion of 18F-FDG PET in current guidelines as an alternative to other nuclear techniques or as a stand-alone investigation may be a consideration [5•].
Taken together, for a definitive diagnosis and management all patients will likely benefit from an echocardiogram and either cardiac MRI or 18F-FDG PET. One could utilize CMR as an initial test in patients with suspected cardiac sarcoidosis. Gadolinium enhanced CMR with delayed imaging offers the advantage of high sensitivity and spatial resolution, in addition to no radiation exposure. If negative, 18F-FDG PET can be avoided. If gadolinium enhancement is seen on CMR and cardiac sarcoidosis is thought to be plausible in the adequate clinical context, 18F-FDG PET imaging could be performed to establish baseline disease activity, assess need for initiation of medical therapy and monitor response to treatment over time. Alternatively, in patients with contraindication to performing CMR, 18F-FDG PET could be used as first line imaging.
Conclusion
The diagnosis of cardiac sarcoidosis remains elusive and subject to various approaches. Early diagnosis is particularly important given that steroid therapy in patients with established cardiac sarcoid and active inflammation should be initiated before left ventricular systolic function declines [38]. Endomyocardial biopsy can be of limited diagnostic yield; in addition to being invasive and posing an associated risk of morbidity, it may lack sensitivity because myocardial involvement is patchy and not homogenous [47]. The life-threatening complications of cardiac sarcoidosis, coupled with the potential benefit of early treatment, have made the utilization of more sensitive imaging modalities crucial in an attempt for early diagnosis and management.
While the revised Japanese Ministry Guidelines published in 2006 are currently the official diagnostic guide and provide an excellent framework to identify patients with cardiac involvement, they utilize techniques that lack sensitivity and specificity. Imaging modalities that can both identify disease activity and potentially predict response to therapy are needed to improve management of this disease. In the last decade alone, 18F-FDG PET has substantially enhanced detection of cardiac sarcoid, and is proving to be a very useful imaging technique in diagnosis, assessing disease activity, monitoring treatment response and risk assessment in patients with sarcoidosis. As data about 8F-FDG PET continues to emerge, there will be a need for standardization of the imaging technique and revision of the diagnostic criteria.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–11.
Nomura S, Funabashi N, Tsubura M, Uehara M, Shiina Y, Daimon M, et al. Cardiac sarcoidosis evaluated by multimodality imaging. Int J Cardiol. 2011;150:e81–4.
Kim JS, Judson MA, Donnino R, Gold M, Cooper Jr LT, Prystowsky EN, et al. Cardiac sarcoidosis. Am Heart J. 2009;157:9–21.
Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest. 1993;103:253–8.
• Youssef G, Beanlands RS, Birnie DH, Nery PB. Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment. Heart. 2011;97:2078–87. Excellent review describing multimodality imaging of cardiac sarcoidosis and its value in diagnosis and management.
•• Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–8. Meta-analysis of 7 studies comparing the accuracy of 18F-FDG PET for the diagnosis of cardiac sarcoidosis.
Matsui Y, Iwai K, Tachibana T, Fruie T, Shigematsu N, Izumi T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–69.
Hiraga H, Hiroe M, Iwai K, et al. Guideline for the diagnosis of cardiac sarcoidosis: study report on Diffuse Pulmonary Diseases (in Japanese). 1993:23–4.
•• Tahara N, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–28. First study to use quantitative assessment of FDG uptake in patients with cardiac sarcoidosis compared with normal and dilated cardiomyopathy patients.
Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis and Granulomatous Disorders (in Japanese). 2007;27:89–102.
Roberts WC, McAllister Jr HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977;63:86–108.
Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138(2 Pt 1):299–302.
Ratner SJ, Fenoglio Jr JJ, Ursell PC. Utility of endomyocardial biopsy in the diagnosis of cardiac sarcoidosis. Chest. 1986;90:528–33.
Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–6.
Takeda N, Yokoyama I, Hiroi Y, Sakata M, Harada T, Nakamura F, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation. 2002;105:1144–5.
•• Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. This is an excellent and comprehensive review covering discussions on etiology, genetics, immunopathogenesis, diagnosis, clinical manifestations, and treatment of sarcoidosis.
Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92:282–8.
Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937–49.
Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, et al. Comparative evaluation of 18F-FDG PET and 67 Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–6.
Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–43.
Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–98.
Pandya C, Brunken RC, Tchou P, Schoenhagen P, Culver DA. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J. 2007;29:418–22.
Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–35.
Newsholme P, Newsholme EA. Rates of utilization of glucose, glutamine and oleate and formation of end-products by mouse peritoneal macrophages in culture. Biochem J. 1989;261:211–8.
Isiguzo M, Brunken R, Tchou P, Xu M, Culver DA. Metabolism-perfusion imaging to predict disease activity in cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:50–5.
Treglia G, Taralli S, Giordano A. Emerging role of whole-body 18F-fluorodeoxyglucose positron emission tomography as a marker of disease activity in patients with sarcoidosis: a systematic review. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:87–94.
Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801–10.
Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–8.
•• Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. Am J Roentgenol. 2008;190:W151–6. One of the seminal papers about the technique to reduce myocardial 18F-FDG uptake.
Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011;18:926–36.
Lum DP, Wandell S, Ko J, Coel MN. Reduction of myocardial 2-deoxy-2-[18F]fluoro-D-glucose uptake artifacts in positron emission tomography using dietary carbohydrate restriction. Mol Imaging Biol. 2002;4:232–7.
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Gropler RJ, et al. PET Myocardial Perfusion and Metabolism Clinical Imaging. J Nucl Cardiol 2009;16. doi:10.1007/s12350-009-9094-9. Available at: http://www.asnc.org/imageuploads/ImagingGuidelinesPETJuly2009.pdf. Accessed 24 Jan 2013.
Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–73.
Blankstein R, Naya M, Osborne M, Kim C, Murthy V, Kwong R, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoid. J Am Coll Cardiol. 2011;59:E1310.
Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86.
Pierre-Louis B, Prasad A, Frishman WH. Cardiac manifestations of sarcoidosis and therapeutic options. Cardiol Rev. 2009;17:153–8.
Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009;20:578–83.
Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–6.
Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–10.
•• Mantini N, Williams Jr B, Stewart J, Rubinsztain L, Kacharava A. Cardiac sarcoid: a clinician's review on how to approach the patient with cardiac sarcoid. Clin Cardiol. 2012;35:410–5. Recent review paper summarizing several current concepts in the diagnosis and managements of patients with cardiac sarcoidosis.
Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–41.
Bargout R, Kelly RF. Sarcoid heart disease: clinical course and treatment. Int J Cardiol. 2004;97:173–82.
••Matthews R, Bench T, Meng H, Franceschi D, Relan N, Brown DL. Diagnosis and monitoring of cardiac sarcoidosis with delayed-enhanced MRI and 18F-FDG PET-CT. J Nucl Cardiol. 2012;19:807–10. Interesting case-report illustrating the differential information provided by 18F-FDG PET and cardiac MRI.
Yazaki Y, Isobe M, Hayasaka M, Tanaka M, Fujii T, Sekiguchi M. Cardiac sarcoidosis mimicking hypertrophic cardiomyopathy: clinical utility of radionuclide imaging for differential diagnosis. Jpn Circ J. 1998;62:465–8.
Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Hear Rhythm. 2012;9:884–91.
Haraki T, Ueda K, Shintani H, Hayashi T, Taki J, Mabuchi H. Spontaneous development of left ventricular aneurysm in a patient with untreated cardiac sarcoidosis. Circ J. 2002;66:519–21.
Deng JC, Baughman RP, Lynch III JP. Cardiac involvement in sarcoidosis. Semin Respir Crit Care Med. 2002;23:513–27.
•• Ohira H, Tsujino I, Yoshinaga K. (1)F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2011;38:1773–83. This reference provides an excellent overview for imaging techniques and patient preparation.
Conflicts of Interest
Hicham Skali declares that he has no conflict of interest.
Allison R. Schulman declares that she has no conflict of interest.
Sharmila Dorbala has received salary support from a research grant from the National Institutes of Health (5K23HL092299-03); has received grant support from Astellas Global Pharma Development, Bracco Diagnostics; has received travel/accommodations expenses covered or reimbursed from RSNA, ASNC.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Skali, H., Schulman, A.R. & Dorbala, S. 18F-FDG PET/CT for the Assessment of Myocardial Sarcoidosis. Curr Cardiol Rep 15, 370 (2013). https://doi.org/10.1007/s11886-013-0370-6
Published:
DOI: https://doi.org/10.1007/s11886-013-0370-6