Abstract
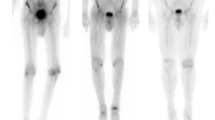
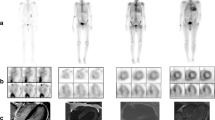
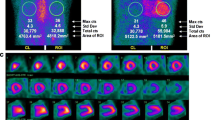
Amyloidosis is a heterogeneous group of diseases with a common feature of extracellular deposition and infiltration of different types of amyloid fibrils in various organs. For example, Alzheimer’s disease is characterized by deposition of amyloid β in the brain. Radiolabeled positron emission tomography (PET) tracers, mainly derivatives of thioflavin-T, were recently introduced for identification of amyloid β plaques in Alzheimer’s patients. Such advances of amyloid β plaque imaging of the brain may shed light into imaging of other organs in amyloidosis patients, such as the heart. Cardiac infiltration of amyloid confers poor clinical outcomes, which renders early diagnosis for appropriate clinical management. At present, nuclear imaging of cardiac amyloidosis is predominantly accomplished with bone-seeking radiotracers, such as 99m-technetium-labeled pyrophosphate (99mTc-PYP), 99m-technetium-methylene diphosphonate (99mTc-MDP), and 99m-technetium-3,3,-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), with conflicting results in terms of diagnostic performance, with the exception for 99mTc-DPD, which may differentiate light-chain amyloidosis from transthyretin-related cardiac amyloidosis. Although other non–bone-seeking radiotracers such as iodine-123-labeled amyloid P component (123I-SAP), 123-iodine-Meta-iodobenzylguanidine (123I-mIBG), 99m-technetium-labeled protease inhibitor, and indium-111-labeled amyloid antibodies have also shown some success in identifying cardiac amyloidosis, the future, however, may lie in labeling derivatives of thioflavin-T. With the recent success of visualizing deposition of amyloid β in the brain, the US Food and Drug Administration–approved PET imaging agent 18F-florbetapir may be used to target cardiac amyloidosis next.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17:101–4.
Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59.
Cornwell GG, Murdoch WL, Kyle RA, et al. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983;75:618–23.
Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–73.
Desai HV, Aronow WS, Peterson SJ, et al. Cardiac amyloidosis: approaches to diagnosis and management. Cardiol Rev. 2010;18:1–11.
Dubrey SW, Cha K, Simms RW, et al. Electrocardiography and Doppler echocardiography in secondary (AA) amyloidosis. Am J Cardiol. 1996;77:313–5.
Biancalana M, Koide S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 2010;1804:1405–12.
Serdons K, Verduyckt T, Vanderghinste D, et al. Synthesis of 18F-labelled 2-(4′-fluorophenyl)-1,3-benzothiazole and evaluation as amyloid imaging agent in comparison with [11C]PIB. Bioorg Med Chem Lett. 2009;19:602–5.
Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19.
Svedberg MM, Hall H, Hellstrom-Lindahl E, et al. [(11)C]PIB-amyloid binding and levels of Abeta40 and Abeta42 in postmortem brain tissue from Alzheimer patients. Neurochem Int. 2009;54:347–57.
Lin KJ, Hsu WC, Hsiao IT, et al. Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent–a pilot study. Nucl Med Biol. 2010;37:497–508.
Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F18). J Nucl Med. 2010;51:913–20. This was an open-label, multicenter brain imaging, metabolism, and safety study of 18F -florbetapir-PET imaging on 16 patients with Alzheimer’s disease (AD) and 16 cognitively healthy controls. The cortical-to-cerebellar standardized uptake value ratios (SUVRs) in AD patients showed continual substantial increases through 30 min after tracer administration, reaching a plateau within 50 min. The cortical average SUVR was significantly higher in AD than controls. The results indicated that 18F -florbetapir was well tolerated, and PET imaging showed significant discrimination between AD and controls.
Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–83. The study prospectively evaluated brain florbetapir-PET imaging in patients from hospice, long-term care facilities near the end of their lives and compared with immunohistochemistry and silver stain measures of brain β-amyloid after their death. Both visual interpretation of the florbetapir-PET images and mean quantitative estimations of cortical uptake were correlated well with presence and quantity of β-amyloid pathology at autopsy as measured by immunohistochemistry and silver stain neuritic plaque score. Florbetapir-PET scans were negative in healthy controls.
Wizenberg TA, Muz J, Sohn YH, et al. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis. Am Heart J. 1982;103:468–73.
Falk RH, Lee VW, Rubinow A, et al. Sensitivity of technetium-99m-pyrophosphate scintigraphy in diagnosing cardiac amyloidosis. Am J Cardiol. 1983;51:826–30.
Gertz MA, Brown ML, Hauser MF, et al. Utility of technetium Tc 99m pyrophosphate bone scanning in cardiac amyloidosis. Arch Intern Med. 1987;147:1039–44.
Eriksson P, Backman C, Bjerle P, et al. Non-invasive assessment of the presence and severity of cardiac amyloidosis. A study in familial amyloidosis with polyneuropathy by cross sectional echocardiography and technetium-99m pyrophosphate scintigraphy. Br Heart J. 1984;52:321–6.
Hongo M, Yamada H, Okubo S, et al. Cardiac involvement in systemic amyloidosis: myocardial scintigraphic evaluation. J Cardiogr. 1985;15:163–80.
Ak I, Vardareli E, Erdinĉ O, et al. Myocardial Tc-99m MDP uptake on a bone scan in senile systemic amyloidosis with cardiac involvement. Clin Nucl Med. 2000;25:826–7.
Lee VW, Caldarone AG, Falk RH, et al. Amyloidosis of heart and liver: comparison of Tc-99m pyrophosphate and Tc-99m methylene diphosphonate for detection. Radiology. 1983;148:239–42.
Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–84.
Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38:470–8. This was a clinical study with the largest cohort of amyloidosis patients in the literature to date (45 patients with ATTR, 34 with AL, and 15 controls) to evaluate the role of 99m Tc-DPD scintigraphy in differentiation of TTR from AL-related cardiac amyloidosis. The results showed that although 99m Tc-DPD was confirmed to be useful for differentiating TTR from AL-related cardiac amyloidosis, the diagnostic accuracy was lower than previously reported in a smaller group of patients (100% sensitivity and accuracy) due to a mild degree tracer uptake in about one third of AL patients. However, 99m Tc-DPD scan can provide an accurate differential diagnosis in cases of absent or intense uptake.
Hawkins PN. Serum amyloid P component scintigraphy for diagnosis and monitoring amyloidosis. Curr Opin Nephrol Hypertens. 2002;11:649–55.
Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323:508–13.
Hazenberg BP, van Rijswijk MH, Lub-de Hooge MN, et al. Diagnostic performance and prognostic value of extravascular retention of 123I-labeled serum amyloid P component in systemic amyloidosis. J Nucl Med. 2007;48:865–72.
Sojan SM, Smyth DR, Tsopelas C, et al. Pharmacokinetics and normal scintigraphic appearance of 99mTc aprotinin. Nucl Med Commun. 2005;26:535–9.
Aprile C, Marinone G, Saponaro R, et al. Cardiac and pleuropulmonary AL amyloid imaging with technetium-99m labelled aprotinin. Eur J Nucl Med. 1995;22:1393–401.
Schaadt BK, Hendel HW, Gimsing P, et al. 99mTc-aprotinin scintigraphy in amyloidosis. J Nucl Med. 2003;44:177–83.
Han S, Chong V, Murray T, et al. Preliminary experience of 99mTc-Aprotinin scintigraphy in amyloidosis. Eur J Haematol. 2007;79:494–500.
Chen W, Cao Q, Dilsizian V. Variation of heart-to-mediastinal ratio in (123)I-mIBG cardiac sympathetic imaging: its affecting factors and potential corrections. Curr Cardiol Rep. 2011;13:132–7.
Nakata T, Shimamoto K, Yonekura S, et al. Cardiac sympathetic denervation in transthyretin-related familial amyloidotic polyneuropathy: detection with iodine-123-MIBG. J Nucl Med. 1995;36:1040–2.
Tanaka M, Hongo M, Kinoshita O, et al. Iodine-123 metaiodobenzylguanidine scintigraphic assessment of myocardial sympathetic innervation in patients with familial amyloid polyneuropathy. J Am Coll Cardiol. 1997;29:168–74.
Hongo M, Urushibata K, Kai R, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J. 2002;144:122–9.
Delahaye N, Dinanian S, Slama MS, et al. Cardiac sympathetic denervation in familial amyloid polyneuropathy assessed by iodine-123 metaiodobenzylguanidine scintigraphy and heart rate variability. Eur J Nucl Med. 1999;26:416–24.
Solomon A, Weiss DT, Wall JS. Immunotherapy in systemic primary (AL) amyloidosis using amyloid-reactive monoclonal antibodies. Cancer Biother Radiopharm. 2003;18:853–60.
Wall JS, Kennel SJ, Paulus M, et al. Radioimaging of light chain amyloid with a fibril-reactive monoclonal antibody. J Nucl Med. 2006;47:2016–24.
Wall JS, Paulus MJ, Gleason S, et al. Micro-imaging of amyloid in mice. Methods Enzymol. 2006;412:161–82.
Wall JS, Kennel SJ, Stuckey AC, et al. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood. 2010;116:2241–4.
Lekakis J, Dimopoulos M, Nanas J, et al. Antimyosin scintigraphy for detection of cardiac amyloidosis. Am J Cardiol. 1997;80:963–5.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Dilsizian, V. Molecular Imaging of Amyloidosis: Will the Heart Be the Next Target After the Brain?. Curr Cardiol Rep 14, 226–233 (2012). https://doi.org/10.1007/s11886-011-0239-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-011-0239-5