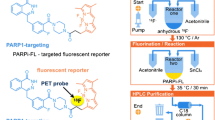
Abstract
Purpose
The current study presents [18F]PARPi as imaging agent for PARP1 expression.
Procedures
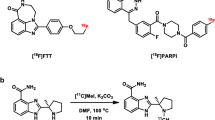
[18F]PARPi was generated by conjugating a 2H-phthalazin-1-one scaffold to 4-[18F]fluorobenzoic acid. Biochemical assays, optical in vivo competition, biodistribution analysis, positron emission tomography (PET)/X-ray computed tomography, and PET/magnetic resonance imaging studies were performed in subcutaneous and orthotopic mouse models of glioblastoma.
Results
[18F]PARPi shows suitable pharmacokinetic properties for brain tumor imaging (IC50 = 2.8 ± 1.1 nM; logPCHI = 2.15 ± 0.41; plasma-free fraction = 63.9 ± 12.6 %) and accumulates selectively in orthotopic brain tumor tissue. Tracer accumulation in subcutaneous brain tumors was 1.82 ± 0.21 %ID/g, whereas in healthy brain, the uptake was only 0.04 ± 0.01 %ID/g.
Conclusions
[18F]PARPi is a selective PARP1 imaging agent that can be used to visualize glioblastoma in xenograft and orthotopic mouse models with high precision and good signal/noise ratios. It offers new opportunities to non-invasively image tumor growth and monitor interventions.






Similar content being viewed by others
References
Rouleau M, Patel A, Hendzel MJ et al (2010) PARP inhibition: PARP1 and beyond. Nat Rev Cancer 10:293–301
Gradwohl G, Menissier de Murcia JM et al (1990) The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc Natl Acad Sci U S A 87:2990–2994
Hassa PO, Hottiger MO (2008) The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci 13:3046–3082
Yang KS, Kohler RH, Landon M et al (2015) Single cell resolution in vivo imaging of DNA damage following PARP inhibition. Sci Rep 5:10129
Ossovskaya V, Koo IC, Kaldjian EP et al (2010) Upregulation of poly(ADP-ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer 1:812–821
Bièche I, De Murcia G, Lidereau R (1996) Poly(ADP-ribose) polymerase gene expression status and genomic instability in human breast cancer. Clin Cancer Res 2:1163–1167
Rojo F, Garcia-Parra J, Zazo S et al (2012) Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann Oncol 23:1156–1164
Alanazi M, Pathan AA, Arifeen Z et al (2013) Association between PARP-1 V762A polymorphism and breast cancer susceptibility in Saudi population. PLoS One 8, e85541
Galia A, Calogero AE, Condorelli RA et al (2012) PARP-1 protein expression in glioblastoma multiforme. Eur J Histochem EJH 56
Barton VN, Donson AM, Kleinschmidt-DeMasters BK et al (2009) PARP1 expression in pediatric central nervous system tumors. Pediatr Blood Cancer 53:1227–1230
Staibano S, Pepe S, Lo Muzio L et al (2005) Poly(adenosine diphosphate-ribose) polymerase 1 expression in malignant melanomas from photoexposed areas of the head and neck region. Hum Pathol 36:724–731
Thurber GM, Reiner T, Yang KS et al (2014) Effect of small-molecule modification on single-cell pharmacokinetics of PARP inhibitors. Mol Cancer Ther 13:986–995
Reiner T, Lacy J, Keliher EJ et al (2012) Imaging therapeutic PARP inhibition in vivo through bioorthogonally developed companion imaging agents. Neoplasia 14:169–177
Carlucci G, Carney B, Brand C et al (2015) Dual-modality optical/PET imaging of PARP1 in glioblastoma. Mol Imaging Biol. doi:10.1007/s11307-015-0858-0
Irwin CP, Portorreal Y, Brand C et al (2014) PARPi-FL–a fluorescent PARP1 inhibitor for glioblastoma imaging. Neoplasia 16:432–440
Keliher EJ, Klubnick JA, Reiner T et al (2014) Efficient acid-catalyzed (18) F/(19) F fluoride exchange of BODIPY dyes. ChemMedChem 9:1368–1373
Keliher EJ, Reiner T, Turetsky A et al (2011) High-yielding, two-step 18F labeling strategy for 18F-PARP1 inhibitors. ChemMedChem 6:424–427
Thurber GM, Yang KS, Reiner T et al (2013) Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun 4:1504
Reiner T, Keliher EJ, Earley S et al (2011) Synthesis and in vivo imaging of a 18F-labeled PARP1 inhibitor using a chemically orthogonal scavenger-assisted high-performance method. Angew Chem Int Ed Engl 50:1922–1925
Zhou D, Chu W, Xu J et al (2014) Synthesis, [(1)(8)F] radiolabeling, and evaluation of poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors for in vivo imaging of PARP-1 using positron emission tomography. Bioorg Med Chem 22:1700–1707
Menear KA, Adcock C, Boulter R et al (2008) 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: a novel bioavailable inhibitor of poly(ADP-ribose) polymerase-1. J Med Chem 51:6581–6591
Reiner T, Earley S, Turetsky A, Weissleder R (2010) Bioorthogonal small-molecule ligands for PARP1 imaging in living cells. Chembiochem 11:2374–2377
Valko K, Bevan C, Reynolds D (1997) Chromatographic hydrophobicity index by fast-gradient RP-HPLC: a high-throughput alternative to log P/log D. Anal Chem 69:2022–2029
Marik J, Sutcliffe JL (2007) Fully automated preparation of n.c.a. 4-[18F]fluorobenzoic acid and N-succinimidyl 4-[18F]fluorobenzoate using a Siemens/CTI chemistry process control unit (CPCU). Appl Radiat Isot 65:199–203
Li X, Link JM, Stekhova S et al (2008) Site-specific labeling of annexin V with F-18 for apoptosis imaging. Bioconjug Chem 19:1684–1688
Chen Y, Zhang L, Hao Q (2013) Olaparib: a promising PARP inhibitor in ovarian cancer therapy. Arch Gynecol Obstet 288:367–374
Murai J, Huang SY, Renaud A et al (2014) Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther 13:433–443
Acknowledgments
The authors thank Dr. Jason S. Lewis and Dr. Edmund J. Keliher for helpful discussions, Dr. Christian Brand and Christopher P. Irwin for help with experiments and Leah Bassity for editing the manuscript. Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Cancer Center Support Grant No 2 P30 CA008748-48, are gratefully acknowledged. NIH Shared Instrumentation Grant No 1S10 OD016207-01, which provided funding support for the purchase of the Inveon PET/CT, is gratefully acknowledged. The authors further thank the Molecular Cytology Core at Memorial Sloan Kettering Cancer Center (P30 CA008748). The authors thank the NIH (K25EB016673 for T.R.), the Brain Tumor Center of Memorial Sloan Kettering Cancer Center (for T.R.), the Center for Molecular Imaging and Nanotechnology (for T.R.), the Clinical and Translational Science Center (CTSC) at Weill Cornell Medical College (NIH/NCATS Grant TL1TR000459 for B.C.), the American-Italian Cancer Foundation (AICF) Post-Doctoral Research Fellowship (for G.C.), the German Research Foundation (for S.K.), as well as the National Science Foundation Integrative Graduate Education and Research Traineeship (IGERT 0965983 at Hunter College) for their generous support.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflicts of interest.
Additional information
Brandon Carney and Giuseppe Carlucci contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
Supporting Information. Detailed experimental procedures, characterization data, tables, and figures provide additional documentation of the studies described in this manuscript. (PDF 30246 kb)
Rights and permissions
About this article
Cite this article
Carney, B., Carlucci, G., Salinas, B. et al. Non-invasive PET Imaging of PARP1 Expression in Glioblastoma Models. Mol Imaging Biol 18, 386–392 (2016). https://doi.org/10.1007/s11307-015-0904-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0904-y