Abstract
Purpose
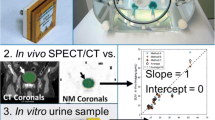
The purpose of this study is to extend an established SPECT/CT quantitation protocol to 177Lu and validate it in vivo using urine samples, thus providing a basis for 3D dosimetry of 177Lu radiotherapy and improvement over current planar methods which improperly account for anatomical variations, attenuation, and overlapping organs.
Procedures
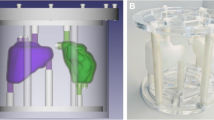
In our quantitation protocol, counts in images reconstructed using an ordered subset-expectation maximization algorithm are converted to kilobecquerels per milliliter using a calibration factor derived from a phantom experiment. While varying reconstruction parameters, we tracked the ratio of image to true activity concentration (recovery coefficient, RC) in hot spheres and a noise measure in a homogeneous region. The optimal parameter set was selected as the point where recovery in the largest three spheres (16, 8, and 4 ml) stagnated, while the noise continued to increase.
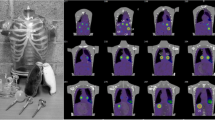
Urine samples were collected following 12 SPECT/CT acquisitions of patients undergoing [177Lu]DOTATATE therapy, and activity concentrations were measured in a well counter. Data was reconstructed using parameters chosen in the phantom experiment, and estimated activity concentration from the images was compared to the urine values to derive RCs.
Results
In phantom data, our chosen parameter set yielded RCs in 16, 8, and 4 ml spheres of 80.0, 74.1, and 64.5 %, respectively. For patients, the mean bladder RC was 96.1 ± 13.2 % (range, 80.6–122.4 %), with a 95 % confidence interval between 88.6 and 103.6 %. The mean error of SPECT/CT concentrations was 10.1 ± 8.3 % (range, −19.4–22.4 %).
Conclusions
Our results show that quantitative 177Lu SPECT/CT in vivo is feasible but could benefit from improved reconstruction methods. Quantifying bladder activity is analogous to determining the amount of activity in the kidneys, an important task in dosimetry, and our results provide a useful benchmark for future efforts.






Similar content being viewed by others
References
Stillebroer AB, Boerman OC, Desar IME et al (2013) Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol 64:478–485
Tagawa ST, Milowsky MI, Morris M et al (2013) Pase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 19:5182–5191
Chakraborty S, Das T, Banerjee S et al (2006) Preparation and preliminary biological evaluation of 177Lu-labelled hydroxyapatite as a promising agent for radiation synovectomy of small joints. Nucl Med Commun 27:661–668
Krenning EP, Kooij PPM, Bakker WH et al (1994) Radiotherapy with a radiolabeled somatostatin analogue, [111In-DTPA-D-Phe1]-octreotide. Ann N Y Acad Sci 733:496–506
de Jong M, Bakker WH, Breeman WAP et al (1998) Pre-clinical comparison of [DPTA0] octreotide, [DTPA0, Tyr3] octreotide and [DOTA0, Tyr3] octreotide as carriers for somatostatin receptor-targeted scintigraphy and radionuclide therapy. Int J Cancer 75:406–411
Valkema R, Pauwels S, Kvols LK et al (2006) Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0, Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 36:147–156
Forrer F, Valkema R, Kwekkeboom DJ et al (2007) Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab 21:111–129
Teunissen JJM, Kwekkeboom DJ, Valkema R et al (2011) Nuclear medicine techniques for the imaging and treatment of neuroendocrine tumours. Endocr Relat Cancer 18:S27–S51
Kwekkeboom DJ, Bakker WH, Kooij PP et al (2001) [177Lu-DOTA0, Tyr3]octreotate: comparison with [111In-DTPA0]octreotide in patients. Eur J Nucl Med Mol Imaging 28:1319–1325
de Jong M, Valkema R, Jamar F et al (2002) Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med 32:133–140
Kwekkeboom DJ, de Herder WW, Kam BL et al (2008) Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 28:2124–2130
Khan S, Krenning EP, van Essen M et al (2011) Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0, Tyr3]octreotate. J Nucl Med 52:1361–1368
Watson EE, Stabin MG, Siegel JA (1993) MIRD formulation. Med Phys 20:511–514
Forrer F, Uusijärvi H, Waldherr C et al (2004) A comparison of 177In-DOTATOC and 111In-DOTATATE: biodistribution and dosimetry in the same patients with metastatic neuroendocrine tumours. Eur J Nucl Med 31(9):1257–1262
Ritt P, Vija H, Hornegger J, Kuwert T (2011) Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging 38:S69–S77
Willowson K, Bailey DL, Baldock C (2008) Quantitative SPECT reconstruction using CT-derived corrections. Phys Med Biol 53:3099–3112
Bailey DL, Willowson KP (2014) Quantitative SPECT/CT: SPECT joins PET as a quantitative imaging modality. Eur J Nucl Med Mol Imaging 41:S17–S25
Sandström M, Garske U, Granberg D et al (2010) Individualized dosimetry in patients undergoing therapy with 177Lu-DOTA-D-Phe1-Tyr3-octreotate. Eur J Nucl Med Mol Imaging 37:212–225
Ichihara T, Ogawa K, Motomura N et al (1993) Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT. J Nucl Med 34:2216–2221
de Nijs R, Lagerburg V, Klausen TL, Holm S (2014) Improving quantitative dosimetry in 177Lu-DOTATATE SPECT by energy window-based scatter corrections. Nucl Med Commun 35:522–533
Vija AH, Hawman EG, Engdahl JC (2003) Analysis of a SPECT OSEM reconstruction method with 3D beam modeling and optional attenuation correction: phantom studies. IEEE Nucl Sci Symp Med Imaging Conf Rec 4:2662–2666
Zeintl J, Vija AH, Yahil A et al (2010) Quantitative accuracy of clinical 99mTc SPECT/CT using ordered-subset expectation maximization with 3-dimensional resolution recovery, attenuation, and scatter correction. J Nucl Med 51:921–928
Beauregard J-M, Hofman MS, Pereira JM et al (2011) Quantitative 177Lu SPECT (QSPECT) imaging using a commercially available SPECT/CT system. Cancer Imaging 11:56–66
Hudson HM, Larkin RS (1994) Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 13:601–609
Dewaraja YK, Frey EC, Sgouros G et al (2012) MIRD Pamphlet no. 23: quantitative SPECT for patient-specific 3-dimensional dosimetry in internal radionuclide therapy. J Nucl Med 53:1310–1325
Chun SY, Fessler JA, Dewaraja YK (2013) Post-reconstruction non-local means filtering methods using CT side information for quantitative SPECT. Phys Med Biol 58:6225–6240
Tsui BMW, Zhao XD, Frey EC (1991) Comparison between ML-EM and WLS-CG algorithms for SPECT image reconstruction. IEEE Trans Nucl Sci 38:1766–1772
Schötzig U, Schrader H, Schönfeld E et al (2001) Standardisation and decay data of 177Lu and 188Re. Appl Radiat Isot 55:89–96
Hutton BF, Hudson HM, Beekman FJ (1997) A clinical perspective of accelerated statistical reconstruction. Eur J Nucl Med Mol Imaging 24:797–808
Shcherbinin S, Piwowarska-Bilska H, Celler A, Birkenfeld B (2012) Quantitative SPECT/CT reconstruction for 177Lu and 177Lu/90Y targeted radionuclide therapies. Phys Med Biol 57:5733–5747
Sorenson JA (1975) Deadtime characteristics of Anger cameras. J Nucl Med 16:284–288
Frey EC, Tsui BMW (1996) A new method for modeling the spatially-variant, object-dependent scatter response function in SPECT. IEEE Nucl Sci Symp Med Imaging Conf Rec 2:1082–1086
Fessler JA, Rogers WL (1996) Spatial resolution properties of penalized-likelihood image reconstruction: space-invariant tomographs. IEEE Trans Image Proc 5:1346–1358
Liow JS, Strother SC (1993) The convergence of object dependent resolution in maximum likelihood based tomographic image reconstruction. Phys Med Biol 38:55–70
Maus J, Hofheinz F, Schramm G et al (2014) Evaluation of PET quantification accuracy in vivo. Nuklearmedizin 53:67–77
Acknowledgments
The authors would like thank our clinic’s technologists and members of the nursing staff, each of whom greatly aided our patient data collection. We also extend our thanks to Michal Cachovan for his thoughtful discussion and technical contributions.
Conflict of Interest
James Sanders, Joachim Hornegger, and Torsten Kuwert have an ongoing research collaboration with Siemens Molecular Imaging in the field of SPECT/CT. Professor Kuwert receives honoraria from Siemens Molecular Imaging for occasional lectures pertaining to SPECT/CT research. Philipp Ritt has no conflict of interest.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethics standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanders, J.C., Kuwert, T., Hornegger, J. et al. Quantitative SPECT/CT Imaging of 177Lu with In Vivo Validation in Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol Imaging Biol 17, 585–593 (2015). https://doi.org/10.1007/s11307-014-0806-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0806-4