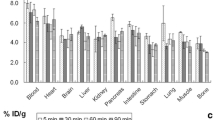
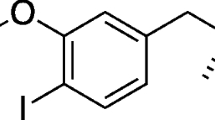
Abstract
Purpose
We aimed to elucidate trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid (anti-[18F]FACBC) uptake mechanisms in inflammatory and tumor cells, in comparison with those of l-[methyl-11C]methionine ([11C]Met) and 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG).
Procedures
Using carbon-14-labeled tracers, in vitro time-course, pH dependence, and competitive inhibition uptake experiments were performed in rat inflammatory (T cells, B cells, granulocytes, macrophages), prostate cancer (MLLB2), and glioma (C6) cells.
Results
Anti-[14C]FACBC uptake ratios of T/B cells to tumor cells were comparable, while those of granulocytes/macrophages to tumor cells were lower than those for [14C]FDG. Over half of anti-[14C]FACBC uptake by T/B and tumor cells was mediated by Na+-dependent amino acid transporters (system ASC), whereas most [14C]Met transport in all cells was mediated by Na+-independent carriers (system L).
Conclusions
The low anti-[18F]FACBC accumulation in granulocytes/macrophages may be advantageous in discriminating inflamed regions from tumors. The significant anti-[18F]FACBC uptake in T/B cells may cause false-positives in some cancer patients who undergo FACBC-positron emission tomography (PET).



Similar content being viewed by others
References
Fanti S, Franchi R, Battista G et al (2005) PET and PET-CT. State of the art and future prospects. Radiol Med 110:1–15
Singhal T, Narayanan TK, Jain V et al (2008) 11C-l-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol 10:1–18
Shoup TM, Olson J, Hoffman JM et al (1999) Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med 40:331–338
Akhurst T, Beattie B, Gogiberidze G, et al. (2006) [18F]FACBC imaging of recurrent gliomas: a comparison with [11C]methionine and MRI [abstract]. J Nucl Med 47:79P.
Oka S, Hattori R, Kurosaki F et al (2007) A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med 48:46–55
Sasajima T, Ono T, Shimada N et al (2013) Trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid (anti-18F-FACBC) is a feasible alternative to 11C-methyl-l-methionine and magnetic resonance imaging for monitoring treatment response in gliomas. Nucl Med Biol 40:808–815
Schuster DM, Taleghani PA, Nieh PT et al (2013) Characterization of primary prostate carcinoma by anti-1-amino-2-[18F] -fluorocyclobutane-1-carboxylic acid (anti-3-[18F] FACBC) uptake. Am J Nucl Med Mol Imaging 3:85–96
Sfanos KS, De Marzo AM (2012) Prostate cancer and inflammation: the evidence. Histopathology 60:199–215
Okudaira H, Shikano N, Nishii R et al (2011) Putative transport mechanism and intracellular fate of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med 52:822–829
Oka S, Okudaira H, Yoshida Y et al (2012) Transport mechanisms of trans-1-amino-3-fluoro[1-14C]cyclobutanecarboxylic acid in prostate cancer cells. Nucl Med Biol 39:109–119
Okudaira H, Nakanishi T, Oka S et al (2013) Kinetic analyses of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid transport in Xenopus laevis oocytes expressing human ASCT2 and SNAT2. Nucl Med Biol 40:670–675
Hediger MA, Romero MF, Peng JB et al (2004) The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Pflugers Arch 447:465–468
Kanai Y, Fukasawa Y, Cha SH et al (2000) Transport properties of a system y+L neutral and basic AA transporter. Insights into the mechanisms of substrate recognition. J Biol Chem 275:20787–20793
Rau FC, Weber WA, Wester HJ et al (2002) O-(2-[18F]Fluoroethyl)-l-tyrosine (FET): a tracer for differentiation of tumour from inflammation in murine lymph nodes. Eur J Nucl Med Mol Imaging 29:1039–1046
Tsukada H, Sato K, Fukumoto D et al (2006) Evaluation of d-isomers of O-11C-methyl tyrosine and O-18F-fluoromethyl tyrosine as tumor-imaging agents in tumor-bearing mice: comparison with l- and d-11C-methionine. J Nucl Med 47:679–688
Tsuyuguchi N, Sunada I, Ohata K et al (2003) Evaluation of treatment effects in brain abscess with positron emission tomography: comparison of fluorine-18-fluorodeoxyglucose and carbon-11-methionine. Ann Nucl Med 17:47–51
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Segel GB, Simon W, Lichtman MA (1984) Multicomponent analysis of amino acid transport in human lymphocytes. Diminished l-system transport in chronic leukemic B lymphocytes. J Clin Invest 74:17–24
Ishii T, Sugita Y, Bannai S (1987) Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J Cell Physiol 133:330–336
Gmünder H, Eck HP, Dröge W (1991) Low membrane transport activity for cystine in resting and mitogenically stimulated human lymphocyte preparations and human T cell clones. Eur J Biochem 201:113–117
Iruloh CG, D'Souza SW, Fergusson WD et al (2009) Amino acid transport systems beta and A in fetal T lymphocytes in intrauterine growth restriction and with tumor necrosis factor-alpha treatment. Pediatr Res 65:51–56
Gaugitsch HW, Prieschl EE, Kalthoff F et al (1992) A novel transiently expressed, integral membrane protein linked to cell activation. Molecular cloning via the rapid degradation signal AUUUA. J Biol Chem 267:11267–11273
Matsumoto Y, Satoh-Ueno K, Yoshimura A et al (1999) Identification and immunological characterization of a novel 40-kDa protein linked to CD98 antigen. Cell Struct Funct 24:217–226
Levring TB, Hansen AK, Nielsen BL et al (2012) Activated human CD4+ T cells express transporters for both cysteine and cystine. Sci Rep 2:266
Fuchs BC, Bode BP (2005) Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 15:254–266
Newsholme P (2001) Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr 131:2515S–2522S
Qu W, Oya S, Lieberman BP et al (2012) Preparation and characterization of l-[5-11C]-glutamine for metabolic imaging of tumors. J Nucl Med 53:98–105
Ploessl K, Wang L, Lieberman BP et al (2012) Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J Nucl Med 53:1616–1624
Shreve PD, Anzai Y, Wahl RL (1999) Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics 19:61–77
Fossati G, Ricevuti G, Edwards SW et al (1999) Neutrophil infiltration into human gliomas. Acta Neuropathol 98:349–354
Kaim AH, Weber B, Kurrer MO et al (2002) Autoradiographic quantification of 18F-FDG uptake in experimental soft-tissue abscesses in rats. Radiology 223:446–451
Zhao S, Kuge Y, Kohanawa M et al (2008) Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: a comparison with 18F-FDG and 18F-FLT. J Nucl Med 49:135–141
Terakawa Y, Tsuyuguchi N, Iwai Y et al (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49:694–699
Ohtsuki S, Uchida Y, Kubo Y, Terasaki T (2011) Quantitative targeted absolute proteomics-based ADME research as a new path to drug discovery and development: methodology, advantages, strategy, and prospects. J Pharm Sci 100:3547–3559
Acknowledgments
The authors thank Mr. Shiro Yoshida and Ms. Sachiko Naito for their assistance in animal treatments and cell cultures, respectively.
Conflict of interest
Shuntaro Oka, Hiroyuki Okudaira, Masahiro Ono, and Yoshifumi Shirakami are employees of Nihon Medi-Physics Co. Ltd. Mark M. Goodman and Emory University have patent rights for anti-[18F]FACBC and are eligible to receive royalties on anti-[18F]FACBC from Nihon Medi-Physics Co. Ltd. Mark M. Goodman, David M. Schuster, and Keiichi Kawai have ongoing research collaborations with Nihon Medi-Physics Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 836 kb)
Rights and permissions
About this article
Cite this article
Oka, S., Okudaira, H., Ono, M. et al. Differences in Transport Mechanisms of trans-1-Amino-3-[18F]Fluorocyclobutanecarboxylic Acid in Inflammation, Prostate Cancer, and Glioma Cells: Comparison with l-[Methyl-11C]Methionine and 2-Deoxy-2-[18F]Fluoro-d-Glucose. Mol Imaging Biol 16, 322–329 (2014). https://doi.org/10.1007/s11307-013-0693-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0693-0