Abstract
Purpose
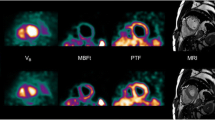
The purpose of this study is to evaluate left ventricular functional parameters in healthy mice and in different murine models of cardiomyopathy with the novel blood pool (BP) positron emission tomography (PET) tracer [68Ga]-albumin.
Procedures
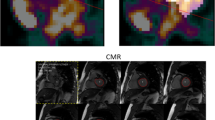
ECG-gated microPET examinations were obtained in healthy mice, and mice with dilative (DCM) and ischemic cardiomyopathy (ICM) using the novel BP tracer [68Ga]-albumin (AlbBP), as well as [18F]-FDG microPET. Cine-magnetic resonance imaging (MRI) examination performed on a clinical 1.5-T MRI provided the reference standard measurements.
Results
When considering the combined group of healthy controls, DCM and ICM AlbBP-PET significantly overestimated the magnitudes of EDV (AlbBP, 181 ± 86 μl; cine-MRI, 125 ± 80 μl; P < 0.001) and ESV (AlbBP, 136 ± 92 μl; cine-MRI, 96 ± 77 μl; P < 0.001), whereas the EF (AlbBP, 31 ± 16 %; cine-MRI, 33 ± 21 %; P = 0.910) matched closely to cine-MRI results, as did findings with [18F]-FDG. High correlations were found between the measured cardiac parameters (EDV: R = 0.978, ESV: R = 0.989, and LVEF: R = 0.992).
Conclusions
Measuring left ventricular function in mice with [68Ga]-albumin BP PET is feasible and showed a high correlation compared to cine-MRI, which was used as a reference standard.






Similar content being viewed by others
References
Brunner S, Todica A, Boning G et al (2012) Left ventricular functional assessment in murine models of ischemic and dilated cardiomyopathy using [18F]FDG-PET: comparison with cardiac MRI and monitoring erythropoietin therapy. EJNMMI Res 2:43
Brunner S, Weinberger T, Huber BC et al (2012) The cardioprotective effects of parathyroid hormone are independent of endogenous granulocyte-colony stimulating factor release. Cardiovasc Res 93:330–339
Brunner S, Zaruba MM, Huber B et al (2008) Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol 36:1157–1166
Huber BC, Brunner S, Segeth A et al (2011) Parathyroid hormone is a DPP-IV inhibitor and increases SDF-1-driven homing of CXCR4(+) stem cells into the ischaemic heart. Cardiovasc Res 90:529–537
Huber BC, Fischer R, Brunner S et al (2010) Comparison of parathyroid hormone and G-CSF treatment after myocardial infarction on perfusion and stem cell homing. Am J Physiol Heart Circ Physiol 298:H1466–1471
Zaruba MM, Huber BC, Brunner S et al (2008) Parathyroid hormone treatment after myocardial infarction promotes cardiac repair by enhanced neovascularization and cell survival. Cardiovasc Res 77:722–731
Lorenz JN, Robbins J (1997) Measurement of intraventricular pressure and cardiac performance in the intact closed-chest anesthetized mouse. Am J Physiol 272:H1137–1146
Tanaka N, Dalton N, Mao L et al (1996) Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 94:1109–1117
Hacker M, Hoyer X, Kupzyk S et al (2006) Clinical validation of the gated blood pool SPECT QBS processing software in congestive heart failure patients: correlation with MUGA, first-pass RNV and 2D-echocardiography. Int J Cardiovasc Imaging 22:407–416
Hacker M, Stork S, Stratakis D et al (2003) Relationship between right ventricular ejection fraction and maximum exercise oxygen consumption: a methodological study in chronic heart failure patients. J Nucl Cardiol 10:644–649
Sibille L, Bouallegue FB, Bourdon A et al (2011) Comparative values of gated blood-pool SPECT and CMR for ejection fraction and volume estimation. Nucl Med Commun 32:121–128
Nichols KJ, Van Tosh A, Wang Y, Palestro CJ, Reichek N (2009) Validation of gated blood-pool SPECT regional left ventricular function measurements. J Nucl Med 50:53–60
Nichols K, Saouaf R, Ababneh AA et al (2002) Validation of SPECT equilibrium radionuclide angiographic right ventricular parameters by cardiac magnetic resonance imaging. J Nucl Cardiol 9:153–160
Kreissl MC, Wu HM, Stout DB et al (2006) Noninvasive measurement of cardiovascular function in mice with high-temporal-resolution small-animal PET. J Nucl Med 47:974–980
Yang Y, Rendig S, Siegel S, Newport DF, Cherry SR (2005) Cardiac PET imaging in mice with simultaneous cardiac and respiratory gating. Phys Med Biol 50:2979–2989
Stegger L, Heijman E, Schafers KP et al (2009) Quantification of left ventricular volumes and ejection fraction in mice using PET, compared with MRI. J Nucl Med 50:132–138
Gropler RJ, Beanlands RS, Dilsizian V et al (2010) Imaging myocardial metabolic remodeling. J Nucl Med 51(1):88S–101S
Higuchi T, Nekolla SG, Jankaukas A et al (2007) Characterization of normal and infarcted rat myocardium using a combination of small-animal PET and clinical MRI. J Nucl Med 48:288–294
Wangler C, Wangler B, Lehner S et al (2011) A universally applicable 68Ga-labeling technique for proteins. J Nucl Med 52:586–591
Deindl E, Zaruba MM, Brunner S et al (2006) G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J 20:956–958
Kandolf R, Hofschneider PH (1985) Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A 82:4818–4822
Rutschow S, Leschka S, Westermann D et al (2010) Left ventricular enlargement in coxsackievirus-B3 induced chronic myocarditis—ongoing inflammation and an imbalance of the matrix degrading system. Eur J Pharmacol 630:145–151
Germano G, Kiat H, Kavanagh PB et al (1995) Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 36:2138–2147
Germano G, Kavanagh PB, Slomka PJ et al (2007) Quantitation in gated perfusion SPECT imaging: the Cedars-Sinai approach. J Nucl Cardiol 14:433–454
Van Kriekinge SD, Berman DS, Germano G (1999) Automatic quantification of left ventricular ejection fraction from gated blood pool SPECT. J Nucl Cardiol 6:498–506
Croteau E, Benard F, Cadorette J et al (2003) Quantitative gated PET for the assessment of left ventricular function in small animals. J Nucl Med 44:1655–1661
Nekolla SG, Miethaner C, Nguyen N, Ziegler SI, Schwaiger M (1998) Reproducibility of polar map generation and assessment of defect severity and extent assessment in myocardial perfusion imaging using positron emission tomography. Eur J Nucl Med 25:1313–1321
Wollenweber T, Zach C, Rischpler C et al (2010) Myocardial perfusion imaging is feasible for infarct size quantification in mice using a clinical single-photon emission computed tomography system equipped with pinhole collimators. Mol Imaging Biol 12:427–434
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Hoffend J, Mier W, Schuhmacher J et al (2005) Gallium-68-DOTA-albumin as a PET blood-pool marker: experimental evaluation in vivo. Nucl Med Biol 32:287–292
Chin BB, Metzler SD, Lemaire A et al (2007) Left ventricular functional assessment in mice: feasibility of high spatial and temporal resolution ECG-gated blood pool SPECT. Radiology 245:440–448
Schinkel AF, Poldermans D, Elhendy A, Bax JJ (2007) Assessment of myocardial viability in patients with heart failure. J Nucl Med 48:1135–1146
Wangler C, Wangler B, Lehner S et al (2011) A universally applicable 68Ga-labeling technique for proteins. J Nucl Med 52:586–591
Jodal L, Le Loirec C, Champion C (2012) Positron range in PET imaging: an alternative approach for assessing and correcting the blurring. Phys Med Biol 57:3931–3943
Disselhorst JA, Brom M, Laverman P et al (2010) Image-quality assessment for several positron emitters using the NEMA NU 4–2008 standards in the Siemens Inveon small-animal PET scanner. J Nucl Med 51:610–617
Cheng JC, Shoghi K, Laforest R (2012) Quantitative accuracy of MAP reconstruction for dynamic PET imaging in small animals. Med Phys 39:1029–1041
Hesse B, Lindhardt TB, Acampa W et al (2008) EANM/ESC guidelines for radionuclide imaging of cardiac function. Eur J Nucl Med Mol Imaging 35:851–885
Acknowledgements
Financial support was provided by the Fritz-Bender-Stiftung and the Else Kröner-Fresenius-Stiftung.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Andrei Todica and Stefan Brunner contributed equally to this work.
Rights and permissions
About this article
Cite this article
Todica, A., Brunner, S., Böning, G. et al. [68Ga]-Albumin-PET in the Monitoring of Left Ventricular Function in Murine Models of Ischemic and Dilated Cardiomyopathy: Comparison with Cardiac MRI. Mol Imaging Biol 15, 441–449 (2013). https://doi.org/10.1007/s11307-013-0618-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0618-y