Abstract
Purpose
We evaluated whether 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) and 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) positron emission tomography (PET) could be used as imaging biomarkers of platinum resensitization in ovarian cancer.
Procedures
Paired platinum-sensitive and platinum-resistant ovarian cancer cells from the same patient, PEO1 and PEO4, grown as tumor xenografts in nude mice, were assessed by PET.
Results
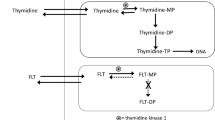
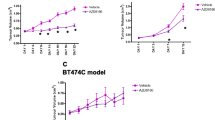
The AKT inhibitor, API-2, resensitized platinum-resistant PEO4 tumors to cisplatin, leading to a markedly lower Ki67 labeling index (p ≤ 0.006, n = 6 per group). [18F]FDG-PET and [18F]FLT-PET imaging variables were lower after combination treatment compared with vehicle treatment (p ≤ 0.006, n = 6 per group). No changes were seen with either drug alone. PRAS40 phosphorylation status was a sensitive biochemical marker of pathway inhibition, whereas reductions thymidine kinase 1 expression defined the [18F]FLT response.
Conclusions
Therapeutic inhibition of AKT activation in acquired platinum-resistant disease can be imaged noninvasively by [18F]FDG-PET and [18F]FLT-PET warranting further assessment.





Similar content being viewed by others
References
Kelland LR (2005) Emerging drugs for ovarian cancer. Expert Opin Emerg Drugs 10:413–424
Kolasa IK, Rembiszewska A, Felisiak A et al (2009) PIK3CA amplification associates with resistance to chemotherapy in ovarian cancer patients. Cancer Biol Ther 8:21–26
Stronach EA, Alfraidi A, Rama N et al (2011) HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res 71:4412–4422
Stronach EA, Chen M, Maginn EN et al (2011) DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired platinum resistance. Neoplasia 13:1069–1108
Langdon SP, Lawrie SS, Hay FG et al (1988) Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res 48:6166–6172
Cooke SL, Ng CKY, Melnyk N et al (2010) Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene 29:4905–4913
Sakai W, Swisher EM, Jacquemont C et al (2009) Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res 69:6381–6386
Workman P, Aboagye EO, Balkwill F et al (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102:1555–1577
Yang L, Dan HC, Sun M et al (2004) Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res 64:4394–4399
Leyton J, Alao JP, Da Costa M et al (2006) In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Cancer Res 66:7621–7629
Leyton J, Latigo JR, Perumal M et al (2005) Early detection of tumor response to chemotherapy by 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo. Cancer Res 65:4202–4210
Leyton J, Smith G, Lees M et al (2008) Noninvasive imaging of cell proliferation following mitogenic extracellular kinase inhibition by PD0325901. Mol Cancer Ther 7:3112–3121
Altomare DA, Zhang L, Deng J et al (2010) GSK690693 delays tumor onset and progression in genetically defined mouse models expressing activated Akt. Clin Cancer Res 16:486–496
Rhodes N, Heerding DA, Duckett DR et al (2008) Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res 68:2366–2374
Workman P, Clarke PA, Guillard S et al (2006) Drugging the PI3 kinome. Nat Biotechnol 24:794–796
Agarwal R, Kaye S (2003) Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer 3:502–516
Liu P, Cheng H, Roberts TM et al (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8:627–644
Stronach EA, Cheraghchi-Bashi A, Chen M et al (2011) Targeting the AKT pathway in ovarian cancer. In: Kaye S, Brown R, Gabra H et al (eds) Emerging therapeutic targets in ovarian cancer. Springer, New York, pp 73–94
Bozulic L, Surucu B, Hynx D et al (2008) PKBalpha/Akt1 acts downstream of DNA–PK in the DNA double-strand break response and promotes survival. Mol Cell 30:203–213
Contractor KB, Aboagye EO (2009) Monitoring predominantly cytostatic treatment response with 18F-FDG PET. J Nucl Med 50:97S–105S
Kenny L, Coombes R, Vigushin D et al (2007) Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging 34:1339–1347
Iagaru AH, Mittra ES, McDougall IR et al (2008) 18F-FDG PET/CT evaluation of patients with ovarian carcinoma. Nucl Med Commun 29:1046–1051
Kurokawa T, Yoshida Y, Kawahara K et al (2004) Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int J Cancer 109:926–932
Kenny LM, Vigushin DM, Al-Nahhas A et al (2005) Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine–positron emission tomography imaging: evaluation of analytical methods. Cancer Res 65:10104–10112
Barthel H, Wilson H, Collingridge DR et al (2004) In vivo evaluation of [18F]fluoroetanidazole as a new marker for imaging tumour hypoxia with positron emission tomography. Br J Cancer 90:2232–2242
Rasey JS, Grierson JR, Wiens LW et al (2002) Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 43:1210–1217
Acknowledgments
The authors thank grant support from Cancer Research UK and Engineering and Physical Sciences Research Council (C2536/A10337), UK Medical Research Council (U1200.005.00001.01) and Ovarian Cancer Action. We would like to thank the Staff at the Biological Imaging Centre at Imperial College for their support.
Potential Conflict of Interest Statement
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM 1
(PDF 36 kb)
Rights and permissions
About this article
Cite this article
Perumal, M., Stronach, E.A., Gabra, H. et al. Evaluation of 2-Deoxy-2-[18F]Fluoro-D-glucose- and 3′-Deoxy-3′-[18F]Fluorothymidine–Positron Emission Tomography as Biomarkers of Therapy Response in Platinum-Resistant Ovarian Cancer. Mol Imaging Biol 14, 753–761 (2012). https://doi.org/10.1007/s11307-012-0554-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-012-0554-2