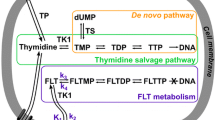
Abstract
Purpose
Longitudinal changes of 3′-[18 F]fluoro-3′-deoxythymidine (FLT) and 2-deoxy-2-[18 F]fluoro-d-glucose (FDG) in response to irinotecan therapy in an animal model of colorectal cancer were compared.
Procedures
SCID/CB-17 mice with HCT116 tumors were treated with 50 mg/kg irinotecan by intraperitoneal injection weekly for 3 weeks. FLT and FDG-positron emission tomography (PET) were performed at baseline, the day after each treatment, and 5 days after the first treatment. Proliferation and apoptosis were evaluated by immunohistochemistry (IHC) after day 15 of imaging.
Results
Irinotecan treatment resulted in a suppression of tumor growth. Tumor FLT uptake was decreased the day after each treatment but to a lesser extent 5 days after the first treatment. FDG uptake increased the day after each treatment with a continuous increase throughout the experiment. IHC analysis of phospho-H3 and Ki67 confirmed FLT-PET results, indicating a decrease in proliferation the day after the final irinotecan treatment. Increased apoptosis monitored by caspase-3 was observed after day 15 with irinotecan treatment.
Conclusions
FLT-PET may be a better method than FDG-PET for assessing treatment response to irinotecan. Changes in imaging occur before changes in tumor volume.




Similar content being viewed by others
References
Shields AF (2006) Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol 8(3):141–150
Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS (2008) Molecular imaging in drug development. Nat Rev Drug Discov 7(7):591–607
Pien HH, Fischman AJ, Thrall JH, Sorensen AG (2005) Using imaging biomarkers to accelerate drug development and clinical trials. Drug Discov Today 10(4):259–266
Bading JR, Shields AF (2008) Imaging of cell proliferation: status and prospects. J Nucl Med 49(Suppl 2):64S–80S
Shields AF, Grierson JR, Dohmen BM et al (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 4(11):1334–1336
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL (2002) Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 43(9):1210–1217
Seitz U, Wagner M, Neumaier B et al (2002) Evaluation of pyrimidine metabolising enzymes and in vitro uptake of 3′-[(18)F]fluoro-3′-deoxythymidine ([(18)F]FLT) in pancreatic cancer cell lines. Eur J Nucl Med Mol Imaging 29(9):1174–1181
Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH (2004) [18 F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging 31(12):1659–1672
Waldherr C, Mellinghoff IK, Tran C et al (2005) Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3′-deoxy-3′-18 F-fluorothymidine PET. J Nucl Med 46(1):114–120
Barthel H, Cleij MC, Collingridge DR et al (2003) 3′-Deoxy-3′-[18 F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res 63(13):3791–3798
Schiepers C, Dahlbom M, Chen W et al (2010) Kinetics of 3′-deoxy-3′-18 F-fluorothymidine during treatment monitoring of recurrent high-grade glioma. J Nucl Med 51(5):720–727
Dittmann H, Dohmen BM, Kehlbach R et al (2002) Early changes in [18 F]FLT uptake after chemotherapy: an experimental study. Eur J Nucl Med Mol Imaging 29(11):1462–1469
Na YS, Jung KA, Kim SM et al (2010) The histone deacetylase inhibitor PXD101 increases the efficacy of irinotecan in in vitro and in vivo colon cancer models. Cancer Chemother Pharmacol 68(2):389–398
Moroz MA, Kochetkov T, Cai S et al (2011) Imaging colon cancer response following treatment with AZD1152: a preclinical analysis of [18 F]fluoro-2-deoxyglucose and 3′-deoxy-3′-[18 F]fluorothymidine imaging. Clin Cancer Res 17(5):1099–1110
Leyton J, Smith G, Lees M et al (2008) Noninvasive imaging of cell proliferation following mitogenic extracellular kinase inhibition by PD0325901. Mol Cancer Ther 7(9):3112–3121
Leyton J, Alao JP, Da Costa M et al (2006) In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18 F]fluorothymidine positron emission tomography. Cancer Res 66(15):7621–7629
Weekes J, Lam AK, Sebesan S, Ho YH (2009) Irinotecan therapy and molecular targets in colorectal cancer: a systemic review. World J Gastroenterol 15(29):3597–3602
Palma JP, Wang YC, Rodriguez LE et al (2009) ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res 15(23):7277–7290
Teicher BA (2002) Tumor models in cancer research, 2nd edn. Humana Press, Totowa
Aide N, Poulain L, Briand M et al (2009) Early evaluation of the effects of chemotherapy with longitudinal FDG small-animal PET in human testicular cancer xenografts: early flare response does not reflect refractory disease. Eur J Nucl Med Mol Imaging 36(3):396–405
Sharma RI, Smith TA (2008) Colorectal tumor cells treated with 5-FU, oxaliplatin, irinotecan, and cetuximab exhibit changes in 18 F-FDG incorporation corresponding to hexokinase activity and glucose transport. J Nucl Med 49(8):1386–1394
Takeba Y, Sekine S, Kumai T et al (2007) Irinotecan-induced apoptosis is inhibited by increased P-glycoprotein expression and decreased p53 in human hepatocellular carcinoma cells. Biol Pharm Bull 30(8):1400–1406
Haberkorn U, Bellemann ME, Brix G et al (2001) Apoptosis and changes in glucose transport early after treatment of Morris hepatoma with gemcitabine. Eur J Nucl Med 28(4):418–425
Takei T, Kuge Y, Zhao S et al (2005) Enhanced apoptotic reaction correlates with suppressed tumor glucose utilization after cytotoxic chemotherapy: use of 99mTc-Annexin V, 18 F-FDG, and histologic evaluation. J Nucl Med 46(5):794–799
Teicher BA (2008) Next generation topoisomerase I inhibitors: Rationale and biomarker strategies. Biochem Pharmacol 75(6):1262–1271
Olivieri G, Micheli A (1983) Mitotic delay and repair in human lymphocytes. Mutat Res 122(1):65–72
Aguda BD (1999) A quantitative analysis of the kinetics of the G(2) DNA damage checkpoint system. Proc Natl Acad Sci U S A 96(20):11352–11357
Furuta M, Hasegawa M, Hayakawa K et al (1997) Rapid rise in FDG uptake in an irradiated human tumour xenograft. Eur J Nucl Med 24(4):435–438
Fishel ML, He Y, Reed AM et al (2008) Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair (Amst) 7(2):177–186
Chen YL, Eriksson S, Chang ZF (2010) Regulation and functional contribution of thymidine kinase 1 in repair of DNA damage. J Biol Chem 285(35):27327–27335
Guichard S, Chatelut E, Lochon I, Bugat R, Mahjoubi M, Canal P (1998) Comparison of the pharmacokinetics and efficacy of irinotecan after administration by the intravenous versus intraperitoneal route in mice. Cancer Chemother Pharmacol 42(2):165–170
Chen W, Delaloye S, Silverman DH et al (2007) Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18 F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol 25(30):4714–4721
Goshen E, Davidson T, Zwas ST, Aderka D (2006) PET/CT in the evaluation of response to treatment of liver metastases from colorectal cancer with bevacizumab and irinotecan. Technol Cancer Res Treat 5(1):37–43
El-Deiry WS, Sigman CC, Kelloff GJ (2006) Imaging and oncologic drug development. J Clin Oncol 24(20):3261–3273
Kelloff GJ, Hoffman JM, Johnson B et al (2005) Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res 11(8):2785–2808
Acknowledgements
The authors thank Velitchka Bontcheva-Diaz, Jerry Clarin, Sally Schlessinger, Lauren Smithee, and Baole Wang for technical assistance and Alan Fan for critical reading of the manuscript. This work was supported by Abbott Laboratories.
Conflict of Interest
All authors are Abbott employees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mudd, S.R., Holich, K.D., Voorbach, M.J. et al. Pharmacodynamic Evaluation of Irinotecan Therapy by FDG and FLT PET/CT Imaging in a Colorectal Cancer Xenograft Model. Mol Imaging Biol 14, 617–624 (2012). https://doi.org/10.1007/s11307-011-0529-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0529-8