Abstract
Purpose
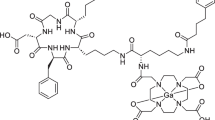
The dimeric transmembrane integrin, αvβ3, is a well-investigated target by different imaging modalities through suitably labeled arginine–glycine–aspartic acid (RGD) containing peptides. In this study, we labeled four cyclic RGD peptides with or without PEG functional groups: c(RGDfK) (denoted as FK), PEG3-c(RGDfK) (denoted as FK-PEG3), E[c(RGDfK)]2 (denoted as [FK]2), and PEG4-E[PEG4-c(RGDfK)]2 (denoted as [FK]2-3PEG4), with 89Zr (t 1/2 = 78.4 h), using the chelator desferrioxamine-p-SCN (Df) for imaging tumor integrin αvβ3.
Methods
The Df conjugated RGD peptides were subjected to integrin αvβ3 binding assay in vitro using MDA-MB-435 breast cancer cells. The 89Zr-labeled RGD peptides were then subjected to small animal positron emission tomography (PET) and direct tissue sampling biodistribution studies in an orthotopic MDA-MB-435 breast cancer xenograft model.
Results
All four tracers, 89Zr-Df-FK, 89Zr-Df-FK-PEG3, 89Zr-Df-[FK]2, and 89Zr-Df-[FK]2-3PEG4, were labeled in high radiochemical yield (89 ± 4%) and high specific activity (4.07–6 MBq/μg). Competitive binding assay with 125I-echistatin showed that conjugation of the RGD peptides to the Df chelator did not have significant impact on their integrin αvβ3 binding affinity and the dimeric peptides were shown to be more potent than the monomers. In agreement with binding results, tumor uptake of 89Zr-Df-[FK]2 and 89Zr-Df-[FK]2-3PEG4 was significantly higher (4.32 ± 1.73%ID/g and 4.72 ± 0.66%ID/g, respectively, at 2 h post-injection) than the monomers 89Zr-Df-FK and 89Zr-Df-FK-PEG3 (1.97 ± 0.38%ID/g and 1.57 ± 0.49%ID/g, respectively, at 2 h post-injection). Out of the four labeled peptides, 89Zr-Df-[FK]2-3PEG4 gave the highest tumor-to-background ratio (18.21 ± 2.52 at 2 h post-injection and 19.69 ± 3.99 at 4 h post-injection), with the lowest uptake in metabolic organs. Analysis of late time points biodistribution data revealed that the uptake in the tumor was decreased, along with increase in the bone, which implies decomplexation of 89Zr-Df.
Conclusion
Efficient radiolabeling of peptides with an appropriate chelator such as Df-RGD with 89Zr was observed. The 89Zr radiolabeled peptides provided high-quality and high-resolution microPET images in xenograft models. 89Zr-Df-[FK]2-3PEG4 demonstrated the highest tumor-to-background ratio of the compounds tested. Preparation of 89Zr peptides to take advantage of the longer half-life is unwarranted due to the relatively rapid clearance from the tumor region of peptide tracers prepared for this study and the increased uptake in the bone of transchelated 89Zr with time (2.0 ± 0.36%ID/g, 24 h post-injection).






Similar content being viewed by others
References
Liu S (2009) Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem 20:2199–2213
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:249–257
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395
Sengupta S, Chattopadhyay N, Mitra A, Ray S, Dasgupta S, Chatterjee A (2001) Role of αvβ3 integrin receptors in breast tumor. J Exp Clin Cancer Res 20:585–590
Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao H et al (2009) Improving tumor-targeting capability and pharmacokinetics of 99mTc-labeled cyclic RGD dimers with PEG4 linkers. Mol Pharm 6:231–245
Chen X, Park R, Hou Y, Khankaldyyan V, Gonzales-Gomez I, Tohme M et al (2004) MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur J Nucl Med Mol Imaging 31:1081–1089
Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR et al (2004) MicroPET imaging of breast cancer αv-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imaging Biol 6:350–359
Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS (2004) Micro-PET imaging of αvβ3-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging 3:96–104
Dijkgraaf I, Beer AJ, Wester HJ (2009) Application of RGD-containing peptides as imaging probes for αvβ3 expression. Front Biosci 14:887–899
Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC et al (2001) αvβ3 and αvβ5 integrin expression in glioma periphery. Neurosurgery 49:380–389, discussion 90
Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA (1992) Involvement of integrin αv gene expression in human melanoma tumorigenicity. J Clin Invest 89:2018–2022
Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM (2004) β3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol 24:2108–2114
Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS et al (2006) Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J Nucl Med 47:113–121
Chen X (2006) Multimodality imaging of tumor integrin αvβ3 expression. Mini Rev Med Chem 6:227–234
Cai W, Niu G, Chen X (2008) Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des 14:2943–2973
Liu S (2006) Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm 3:472–487
Bogler O, Mikkelsen T (2003) Angiogenesis in glioma: molecular mechanisms and roadblocks to translation. Cancer J 9:205–213
Hwang R, Varner J (2004) The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am 18:991–1006, vii
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G et al (1994) Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157–1164
Brooks PC, Clark RA, Cheresh DA (1994) Requirement of vascular integrin αvβ3 for angiogenesis. Science 264:569–571
Dayton PA, Pearson D, Clark J, Simon S, Schumann PA, Zutshi R et al (2004) Ultrasonic analysis of peptide- and antibody-targeted microbubble contrast agents for molecular imaging of αvβ3-expressing cells. Mol Imaging 3:125–134
Ellegala DB, Leong-Poi H, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME et al (2003) Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to αvβ3. Circulation 108:336–341
Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR (2003) Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to αv-integrins. Circulation 107:455–460
Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC (1998) Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat Med 4:623–626
Chen X, Conti PS, Moats RA (2004) In vivo near-infrared fluorescence imaging of integrin αvβ3 in brain tumor xenografts. Cancer Res 64:8009–8014
Chen X, Park R, Shahinian AH, Tohme M, Khankaldyyan V, Bozorgzadeh MH et al (2004) 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol 31:179–189
Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS (2004) MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem 15:41–49
Chen X, Park R, Shahinian AH, Bading JR, Conti PS (2004) Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl Med Biol 31:11–19
Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I et al (2004) Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor αvβ3-integrin expression. J Nucl Med 45:1776–1783
Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL et al (2001) Noninvasive imaging of αvβ3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res 61:1781–1785
Haubner R, Bruchertseifer F, Bock M, Kessler H, Schwaiger M, Wester HJ (2004) Synthesis and biological evaluation of a 99mTc-labelled cyclic RGD peptide for imaging the αvβ3 expression. Nuklearmedizin 43:26–32
Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester HJ et al (2004) [18F]Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem 15:61–69
Li ZB, Chen K, Chen X (2008) 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αvβ3 expression. Eur J Nucl Med Mol Imaging 35:1100–1108
Wu Z, Li ZB, Chen K, Cai W, He L, Chin FT et al (2007) microPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4). J Nucl Med 48:1536–1544
Holland JP, Sheh Y, Lewis JS (2009) Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol 36:729–739
Vosjan MJ, Perk LR, Visser GW, Budde M, Jurek P, Kiefer GE et al (2010) Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc 5:739–743
Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S et al (2005) microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med 46:1707–1718
Janssen M, Oyen WJ, Massuger LF, Frielink C, Dijkgraaf I, Edwards DS et al (2002) Comparison of a monomeric and dimeric radiolabeled RGD-peptide for tumor targeting. Cancer Biother Radiopharm 17:641–646
Haubner R, Wester HJ, Reuning U, Senekowitsch-Schmidtke R, Diefenbach B, Kessler H et al (1999) Radiolabeled αvβ3 integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med 40:1061–1071
Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592
Tinianow JN, Gill HS, Ogasawara A, Flores JE, Vanderbilt AN, Luis E et al (2010) Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl Med Biol 37:289–297
Hoeben BA, Kaanders JH, Franssen GM, Troost EG, Rijken PF, Oosterwijk E et al (2010) PET of hypoxia with 89Zr-labeled cG250-F(ab′)2 in head and neck tumors. J Nucl Med 51:1076–1083
Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS (2010) 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med 51:1293–1300
Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S (2009) Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjug Chem 20:750–759
Meijs WE, Herscheid JDM, Haisma H, Pinedo HM (1992) Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Appl Radiat Isot 43:1443–1447
Acknowledgment
This research was supported by the Intramural Research Program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
Conflict of Interest Statement
Authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobson, O., Zhu, L., Niu, G. et al. MicroPET Imaging of Integrin αvβ3 Expressing Tumors Using 89Zr-RGD Peptides. Mol Imaging Biol 13, 1224–1233 (2011). https://doi.org/10.1007/s11307-010-0458-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0458-y