Abstract
Purpose
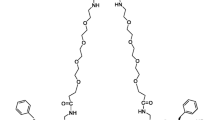
99mTc-3PRGD2 is a 99mTc-labeled dimeric cyclic RGD peptide with increased receptor binding affinity and improved kinetics for in vivo imaging of integrin αvβ3 expression in nude mouse model. To accelerate its clinical translation, we reported here the evaluation of the kit-formulated 99mTc-3PRGD2 in healthy cynomolgus primates for its blood clearance kinetics, biodistribution, and radiation dosimetry.
Procedures
Healthy cynomolgus primates (4.1 ± 0.7 kg, n = 5) were anesthetized, and the venous blood samples were collected via a femoral vein catheter at various time points after injection of ~555 MBq of 99mTc-3PRGD2. Serial whole-body scans were performed with a dual-head single photon emission computed tomography system after administering ~555 MBq of 99mTc-3PRGD2 in the non-human primates, and the radiation dosimetry estimate was calculated.
Results
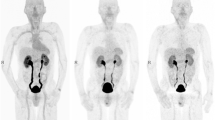
99mTc-3PRGD2 could be easily obtained from freeze-dried kits with high radiochemical purity (>95%) and high specific activity (~5 Ci/μmol). 99mTc-3PRGD2 had a rapid blood clearance with less than 1% of the initial radioactivity remaining in the blood circulation at 60 min postinjection. No adverse reactions were observed up to 4 weeks after the repeated dosing. The whole-body images exhibited high kidney uptake of 99mTc-3PRGD2 and high radioactivity accumulation in the bladder, demonstrating the rapid renal clearance of this tracer. The highest radiation doses of 99mTc-3PRGD2 were found in the kidneys (13.2 ± 1.08 μGy/MBq) and the bladder wall (33.1 ± 1.91 μGy/MBq).
Conclusion
99mTc-3PRGD2 can be readily available using the kit formulation. This tracer is safe and well tolerated, and no adverse events occurred in non-human primates. Further clinical testing and translation of 99mTc-3PRGD2 for noninvasive imaging of integrin αvβ3 in humans are warranted.





Similar content being viewed by others
References
Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25
Brooks PC, Clark RA, Cheresh DA (1994) Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264:569–571
Cai W, Chen X (2008) Multimodality molecular imaging of tumor angiogenesis. J Nucl Med 49(Suppl 2):113S–128S
Cai W, Niu G, Chen X (2008) Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des 14:2943–2973
Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, Schnell O, Niemeyer M, Kessler H, Wester HJ et al (2006) Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin αvβ3 expression in man. Clin Cancer Res 12:3942–3949
Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, Becker KF, Goebel M, Hein R, Wester HJ et al (2005) Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med 2:e70
Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, Spinks TJ, McParland B, Cohen PS, Hui AM, Palmieri C et al (2008) Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med 49:879–886
Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao H, Liu Z, Wang F, Chen X, Liu S (2009) Improving tumor-targeting capability and pharmacokinetics of 99mTc-labeled cyclic RGD dimers with PEG4 linkers. Mol Pharm 6:231–245
Liu Z, Liu S, Wang F, Liu S, Chen X (2009) Noninvasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur J Nucl Med Mol Imaging 36:1296–1307
Liu Z, Niu G, Shi J, Liu S, Wang F, Liu S, Chen X (2009) 68 Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin αvβ3 PET imaging. Eur J Nucl Med Mol Imaging 36:947–957
Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S (2009) Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjug Chem 20:750–759
Liu S (2009) Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjugate Chem 20:2199–2213
Beer AJ, Haubner R, Wolf I, Goebel M, Luderschmidt S, Niemeyer M, Grosu AL, Martinez MJ, Wester HJ, Weber WA et al (2006) PET-based human dosimetry of [18F]Galacto-RGD, a new radiotracer for imaging αvβ3 expression. J Nucl Med 47:763–769
Kowalsky RJ, Falen SW (2004) Radiopharmaceuticals in nuclear pharmacy and nuclear medicine, 2nd edn. American Pharmacist Association, Washington, D.C., pp 71–98
Acknowledgment
This work was supported, in part, by NSFC projects (30870728, 20820102035, 30930030, and 30900373), an “863” project (2007AA02Z467) and grants from the Ministry of Science and Technology of China (2009ZX09103-733, 2009ZX09103-746 and 2009ZX09301-010).
Conflict of interest disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Significance:
99mTc-3PRGD has been reported as a new dimeric Arg-Gly-Asp (RGD) radiotracer with increased receptor binding affinity and improved kinetics for in vivo imaging of integrin αvβ3 expression in nude mouse model. To accelerate its clinical translation, the evaluation of a kit-formulated 99mTc-3PRGD2 in healthy cynomolgus primates for its blood clearance kinetics, biodistribution, and radiation dosimetry was carried out in this paper.
Bing Jia and Zhaofei Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jia, B., Liu, Z., Zhu, Z. et al. Blood Clearance Kinetics, Biodistribution, and Radiation Dosimetry of a Kit-Formulated Integrin αvβ3-Selective Radiotracer 99mTc-3PRGD2 in Non-Human Primates. Mol Imaging Biol 13, 730–736 (2011). https://doi.org/10.1007/s11307-010-0385-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0385-y