Abstract
Purpose
Studies have established the value of [(methyl)1-11C]-acetate ([11C]Act) combined with 2-deoxy-2[18F]fluoro-d-glucose (FDG) for detecting hepatocellular carcinoma (HCC) using positron emission tomography (PET). In this study, the metabolic fate of [11C]Act in HCC was characterized.
Methods
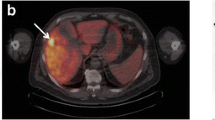
Experiments with acetic acid [1-14C] sodium salt ([14C]Act) were carried out on WCH-17 cells and freshly derived rat hepatocytes. PET scans with [11C]Act were also carried out on woodchucks with HCC before injection of [14C]Act. The radioactivity levels in different metabolites were quantified with thin-layer chromatography.
Results
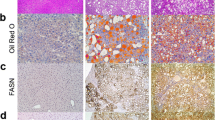
In WCH-17 cells, the predominant metabolite was phosphatidylcholine (PC). Regions of HCCs with the highest [11C]Act uptake had higher radioactivity accumulation in lipid-soluble compounds than surrounding hepatic tissues. In those regions, PC and triacylglycerol (TG) accumulated more radioactivity than in surrounding hepatic tissues.
Conclusions
High [11C]Act uptake in HCC is associated with increased de novo lipogenesis. PC and TG are the main metabolites into which the radioactive label from [11C]Act is incorporated in HCC.





Similar content being viewed by others
References
Ferlay J, Bray F, Pisani P et al (2004) GLOBOCAN 2: cancer incidence, mortality and prevalence worldwide. IARC, Lyon
Theakston F (ed) (2008) World Health Statistics 2008. World Health Organization, Geneva, p 112
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340(10):745–750
El-Serag HB, Mason AC (2000) Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med 160(21):3227–3230
El-Serag HB, Mason AC, Key C (2001) Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology 33(1):62–65
Previsani N, Lavanchy D (2002) Hepatitis B. World Health Organization—Department of Communicable Diseases Surveillance and Response, Geneva, p 76
Shin JA, Park JW, An M et al (2006) Diagnostic accuracy of 18F-FDG positron emission tomography for evaluation of hepatocellular carcinoma. Korean J Hepatol 12(4):546–552
Yamamoto Y, Nishiyama Y, Kameyama R et al (2008) Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med 49(8):1245–1248
Lin WY, Tsai SC, Hung GU (2005) Value of delayed 18F-FDG-PET imaging in the detection of hepatocellular carcinoma. Nucl Med Commun 26(4):315–321
Wudel LJ Jr, Delbeke D, Morris D et al (2003) The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am Surg 69(2):117–124; discussion 124–116
Trojan J, Schroeder O, Raedle J et al (1999) Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol 94(11):3314–3319
Delbeke D, Martin WH, Sandler MP et al (1998) Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg 133(5):510–515; discussion 515–516
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42(5):1208–1236
Llovet JM, Bruix J (2008) Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 48(Suppl 1):S20–S37
Ho CL, Yu SC, Yeung DW (2003) 11C-Acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med 44(2):213–221
Li S, Beheshti M, Peck-Radosavljevic M et al (2006) Comparison of (11)C-acetate positron emission tomography and (67)Gallium citrate scintigraphy in patients with hepatocellular carcinoma. Liver Int 26(8):920–927
Park JW, Kim JH, Kim SK et al (2008) A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med 49(12):1912–1921
Hwang KH, Choi DJ, Lee SY et al (2009) Evaluation of patients with hepatocellular carcinomas using [(11)C]acetate and [(18)F]FDG PET/CT: a preliminary study. Appl Radiat Isot 67:1195–1198
Yu MC, Yuan JM, Govindarajan S et al (2000) Epidemiology of hepatocellular carcinoma. Can J Gastroenterol 14(8):703–709
Tennant BC, Toshkov IA, Peek SF et al (2004) Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology 127(5 Suppl 1):S283–S293
Abe K, Kurata T, Shikata T et al (1988) Enzyme-altered liver cell foci in woodchucks infected with woodchuck hepatitis virus. Jpn J Cancer Res 79(4):466–472
Aldrich CE, Coates L, Wu TT et al (1989) In vitro infection of woodchuck hepatocytes with woodchuck hepatitis virus and ground squirrel hepatitis virus. Virology 172(1):247–252
Chen HS, Kaneko S, Girones R et al (1993) The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J Virol 67(3):1218–1226
Cote PJ, Korba BE, Steinberg H et al (1991) Cyclosporin A modulates the course of woodchuck hepatitis virus infection and induces chronicity. J Immunol 146(9):3138–3144
Korba BE, Cote PJ, Wells FV et al (1989) Natural history of woodchuck hepatitis virus infections during the course of experimental viral infection: molecular virologic features of the liver and lymphoid tissues. J Virol 63(3):1360–1370
Popper H, Roth L, Purcell RH et al (1987) Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci U S A 84(3):866–870
Chu CK, Boudinot FD, Peek SF et al (1998) Preclinical investigation of L-FMAU as an anti-hepatitis B virus agent. Antivir Ther 3(Suppl 3):113–121
Luxembourg A, Hannaman D, Wills K et al (2008) Immunogenicity in mice and rabbits of DNA vaccines expressing woodchuck hepatitis virus antigens. Vaccine 26(32):4025–4033
Menne S, Butler SD, George AL et al (2008) Antiviral effect of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate, administered orally alone and in combination, to woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother 52: 3617–3632
Menne S, Cote PJ, Korba BE et al (2005) Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother 49(7):2720–2728
Menne S, Tennant BC, Gerin JL et al (2007) Chemoimmunotherapy of chronic hepatitis B virus infection in the woodchuck model overcomes immunologic tolerance and restores T-cell responses to pre-S and S regions of the viral envelope protein. J Virol 81(19):10614–10624
Torizuka T, Tamaki N, Inokuma T et al (1995) In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med 36(10):1811–1817
Salem N, MacLennan GT, Kuang Y et al (2007) Quantitative evaluation of 2-deoxy-2[F-18]fluoro-D-glucose-positron emission tomography imaging on the woodchuck model of hepatocellular carcinoma with histological correlation. Mol Imaging Biol 9(3):135–143
Salem N, Kuang Y, Wang F et al (2009) PET imaging of hepatocellular carcinoma with 2-deoxy-2[18F]fluoro-D-glucose, 6-deoxy-6[18F] fluoro-D-glucose, [1-11C]-acetate and [N-methyl-11C]-choline. Q J Nucl Med Mol Imaging 53(2):144–156
Berry MN, Friend DS (1969) High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol 43(3):506–520
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Smillie RM, Krotkov G (1960) The estimation of nucleic acids in some algae and higher plants. Can J Bot 38:31–49
Yoshimoto M, Waki A, Yonekura Y et al (2001) Characterization of acetate metabolism in tumor cells in relation to cell proliferation: acetate metabolism in tumor cells. Nucl Med Biol 28(2):117–122
Norfolk E, Khan SH, Fried B et al (1994) Comparison of amino acid separations on high performance silica gel, cellulose, and C-18 reversed phase layers and application of HPTLC to the determination of amino acids in Biomphalaria glabrata snails. J Liq Chromatogr Relat Technol 17(6):1317–1326
Kupke IR, Zeugner S (1978) Quantitative high-performance thin-layer chromatography of lipids in plasma and liver homogenates after direct application of 0.5-microliter samples to the silica-gel layer. J Chromatogr 146(2):261–271
Kihlberg T, Valind S, Langstrom B (1994) Synthesis of [1-11C], [2-11C], [1-11C](2H3) and [2-11C](2H3)acetate for in vivo studies of myocardium using PET. Nucl Med Biol 21(8):1067–1072
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509
Brown M, Marshall DR, Sobel BE et al (1987) Delineation of myocardial oxygen utilization with carbon-11-labeled acetate. Circulation 76(3):687–696
Blouin A, Bolender RP, Weibel ER (1977) Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol 72(2):441–455
Jiang J, Xu N, Zhang X et al (2007) Lipids changes in liver cancer. Journal of Zhejiang University-Science B 8(6):398–409
Yamashita T, Honda M, Takatori H et al (2009) Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol 50(1):100–110
Kawata S, Takaishi K, Nagase T et al (1990) Increase in the active form of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human hepatocellular carcinoma: possible mechanism for alteration of cholesterol biosynthesis. Cancer Res 50(11):3270–3273
Yun M, Bang SH, Kim JW et al (2009) The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. J Nucl Med 50(8):1222–1228
Wang F, Anderson PW, Salem N et al (2006) Gene expression studies of hepatitis virus-induced woodchuck hepatocellular carcinoma in correlation with human results. Int J Oncol 30(1):33–44
Yu K, Schomisch SJ, Chandramouli V et al (2006) Hexokinase and glucose-6-phosphatase activity in woodchuck model of hepatitis virus-induced hepatocellular carcinoma. Comp Biochem Physiol C Toxicol Pharmacol 143(2):225–231
Acknowledgments
We are grateful to Dr. Ann-Marie Broome for her technical assistance in obtaining, preserving, and maintaining the cell lines used in this study. This work was supported in part by NIH/NCI CA095307 (Principal Investigator: Zhenghong Lee), a NIH Interdisciplinary Biomedical Imaging Training Program predoctoral training grant T32EB007509-02 (Principal Investigator: David L. Wilson), and a 2008 Society of Nuclear Medicine fellowship award (recipient: Nicolas Salem). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nicolas Salem and Yu Kuang equally contributed to the work presented in this manuscript.
This work was supported by NIH/NCI CA095307, NIH T32007509, and a 2008 SNM fellowship award.
Rights and permissions
About this article
Cite this article
Salem, N., Kuang, Y., Corn, D. et al. [(Methyl)1-11C]-Acetate Metabolism in Hepatocellular Carcinoma. Mol Imaging Biol 13, 140–151 (2011). https://doi.org/10.1007/s11307-010-0308-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0308-y