Abstract
Background
3′-[F-18]Fluoro-3′-deoxythymidine (FLT) traces thymidine phosphorylation catalyzed by thymidine kinase during cell proliferation. Knowing the rate of cell proliferation during cancer treatment, such as radiation therapy, would be valuable in assessing whether tumor recurrence is likely and might indicate the need for additional treatments. However, the relationship between FLT kinetics and the effects of radiation is not well-understood. Nor has the method for optimal quantification of FLT uptake within the irradiated tumor microenvironment been extensively examined.
Materials and Methods
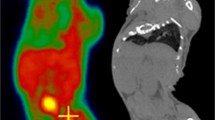
We performed dynamic FLT-positron emission tomography (PET) studies (60 min) on 22 mice implanted subcutaneously with syngeneic mammary MCaK tumors bilaterally in the shoulder area. A day before the FLT-PET imaging, the tumor on the right side was irradiated with a single dose (0, 2.5, 5, 10, or 20 Gy) or with fractionated exposures (4 × 2.5 Gy given in 12 h intervals). Standardized uptake value (SUVs) of FLT on tumors at 10 and 60 min post injection were calculated; model fitting was used to estimate the kinetic parameters. Significant radiation-induced changes were shown by comparing the irradiated tumor with the control tumor in the same animal and by comparing it to nonirradiated mice. The effect of radiation on MCaK cell cycle parameters and FLT uptake was also examined in vitro.
Results
In vivo FLT kinetics were sensitive to radiation doses of 5 Gy and higher (administered 1 day earlier), as judged by SUV semiquantitative measures and by modeling. Single irradiation with 10 Gy had greater impact on SUVs and kinetic parameters than fractionated exposures. Overall, the uptake constant K i appeared to be the best marker for these radiation effects. FLT uptake by irradiated cells in vitro at various doses gave similar findings, and the in vitro FLT uptake correlated well with K i . Radiation-induced G2/M arrest appeared to influence FLT uptake, and this was more pronounced after single than fractionated doses.
Conclusion
The kinetics of FLT uptake into murine mammary tumors was altered 1 day after radiation treatment. The dose-dependent response correlated well with in vitro FLT cellular uptake. Parameters (e.g., K i ) derived from FLT kinetics are expected to be useful for assessing the efficacy of irradiation treatment of tumors.




Similar content being viewed by others
References
Boothman DA, Davis TW, Sahijdak WM (1994) Enhanced expression of thymidine kinase in human cells following ionizing radiation. Int J Radiat Oncol Biol Phys 30:391–398
Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH (2004) [18F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging 31:1659–1672
Kong XB, Zhu QY, Vidal PM et al (1992) Comparisons of anti-human immunodeficiency virus activities, cellular transport, and plasma and intracellular pharmacokinetics of 3¢-fluoro-3¢-deoxythymidine and 3¢-azido-3¢-deoxythymidine. Antimicrob Agents Chemother 36:808–818
Vesselle H, Grierson J, Muzi M et al (2002) In vivo validation of 3¢deoxy-3¢-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 8:3315–3323
Buck AK, Halter G, Schirrmeister H et al (2003) Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med 44:1426–1431
Buck AK, Schirrmeister H, Hetzel M et al (2002) 3-deoxy-3-[(18)F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res 62:3331–3334
Mathews MB, Bernstein RM, Franza BR Jr, Garrels JI (1984) Identity of the proliferating cell nuclear antigen and cyclin. Nature 309:374–376
Shields AF, Grierson JR, Dohmen BM et al (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 4:1334–1336
Mankoff DA, Shields AF, Krohn KA (2005) PET imaging of cellular proliferation. Radiol Clin North Am 43:153–167
Waldherr C, Mellinghoff IK, Tran C et al (2005) Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3¢-deoxy-3¢-18F-fluorothymidine PET. J Nucl Med 46:114–120
Barthel H, Cleij MC, Collingridge DR et al (2003) 3¢-deoxy-3¢-[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res 63:3791–3798
Dittmann H, Dohmen BM, Kehlbach R et al (2002) Early changes in [18F]FLT uptake after chemotherapy: an experimental study. Eur J Nucl Med Mol Imaging 29:1462–1469
Oyama N, Ponde DE, Dence C, Kim J, Tai YC, Welch MJ (2004) Monitoring of therapy in androgen-dependent prostate tumor model by measuring tumor proliferation. J Nucl Med 45:519–525
Schoder H, Gonen M (2007) Screening for cancer with PET and PET/CT:potential and limitations. J Nucl Med 48:4S–18S
Cook G, Maisey MN, Fogelman I (1999) Normal variants, artefacts and interpretative pitfalls in PET imaging with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Eur J Nucl Med 26:1363–1378
Molthoff CFM, Klabbers BM, Berkhof J et al (2007) Monitoring response to radiotherapy in human squamous cell cancer bearing nude mice: comparison of 2-deoxy-2-[18F]fluoro-D-glucose (FDG) and 3-[18F]fluoro-3-deoxythymidine (FLT). Mol Imaging Biol 9:340–347
Kubota K, Ishiwata K, Kubota R et al (1991) Tracer feasibility for monitoring tumor radiotherapy: a quadruple tracer study with fluorine-18-fluorodeoxyglucose or fluorine-18-fluorodeoxyuridine, L-[methyl-14C]methionine, [6-3H]thymidine, and gallium-67. J Nucl Med 32:2118–2123
Zasadny K, Wahl RL (1993) Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variation with body weight and a method for correction. Radiology 189:847–850
Keyes JJ (1995) SUV: standard uptake or silly useless value? J Nucl Med 36:1836–1839
Huang S (2000) Anatomy of SUV. Nucl Med Biol 27:643–646
Phelps ME (2004) PET: molecular imaging and its biological applications. Springer, New York
Muzi M, Vesselle H, Grierson JR et al (2005) Kinetic analysis of 3′-deoxy-3′-fluorothymidine PET studies: validation studies in patients with lung cancer. J Nucl Med 46:274–282
Satyamurthy N, Amarasekera B, Alvord CW, Barrio JR, Phelps ME (2002) Tantalum [18O]water target for the production of [18F]fluoride with high reactivity for the preparation of 2-deoxy-2-[18F]fluoro-D-glucose. Mol Imaging Biol 4:65–70
Pollack A, Terry NH, White RA, Cao S, Meistrich ML, Milas L (1994) Proliferation kinetics of recruited cells in a mouse mammary carcinoma. Cancer Res 54:811–817
Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2:131–137
Muzi M, Mankoff DA, Grierson JR, Wells JM, Vesselle H, Krohn KA (2005) Kinetic modeling of 3¢-deoxy-3¢-fluorothymidine in somatic tumors: mathematical studies. J Nucl Med 46:371–380
Huang SC, Phelps ME, Hoffman EJ, Sideris K, Selin CJ, Kuhl DE (1980) Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol 238:E69–E82
Phelps ME, Huang SC, Hoffman EJ, Selin CE, Kuhl DE (1979) Tomographic measurement of regional cerebral glucose metabolic rate in man with (F-18) fluorodeoxyglucose: validation of method. Ann Neurol 6:371–388
Sokoloff L, Reivich M, Kennedy C et al (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Mankoff DA, Shields AF, Graham MM, Link JM, Eary JF, Krohn KA (1998) Kinetic analysis of 2-[carbon-11]thymidine PET imaging studies: compartmental model and mathematical analysis. J Nucl Med 39:1043–1055
Lee JR, Madsen MT, Bushnel D, Menda Y (2000) A threshold method to improve standardized uptake value reproducibility. Nucl Med Commun 21:685–690
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL (2002) Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 43:1210–1217
Ellims PH, Van der Weyden MB, Medley G (1981) Thymidine kinase isoenzymes in human malignant lymphoma. Cancer Res 41:691–695
Larson SM, Weiden PL, Grunbaum Z et al (1981) Positron imaging feasibility studies. I: characteristics of [3H]thymidine uptake in rodent and canine neoplasms: concise communication. J Nucl Med 22:869–874
Jeong MH, Jin YH, Kang EY, Jo WS, Park HT, Lee JD, Yoo YJ, Jeong SJ (2004) The modulation of radiation-induced cell death by genistein in K562 cells: activation of thymidine kinase 1. Cell Res 14:295–302
Hoffman EJ, Huang SC, Phelps ME (1979) Quantitation in positron emission computed tomography. 1. Effect of object size. J Comput Assist Tomogr 3:299–308
Demaria S, Ng B, Devitt ML et al (2004) Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 58:862–870
Hamada N, Matsumoto H, Hara T, Kobayashi Y (2007) Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. J Radiat Res (Tokyo) 48:87–95
Patlak C, Blasberg RG (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3:1–7
Soret M, Bacharach SL, Buvat I (2007) Partial-volume effect in PET tumor imaging. J Nucl Med 48:932–945
Wu HM, Sui G, Lee CC, Prins ML, Ladno W, Lin HD, Yu AS, Phelps ME, Huang SC (2007) In vivo quantitation of glucose metabolism in mice using microPET imaging and a microfluidic device. J Nucl Med 48:837–845
Huang SC, Wu HM, Kreissl M, Stout D, Chatziioannou A, Truong D, Schelbert HR (2005) Evaluation of partial volume effects in mouse cardiac MicroPET images using a 4D digital mouse phantom. Mol Imaging Biol 7:134
Ferl GZ, Wu HM, Zhang X, Huang SC (2007) Estimation of the 18F-FDG Input function in mice using dynamic microPET and minimal blood sample data. J Nucl Med 48:2037–2045
Acknowledgement
The authors would like to thank Dr. Arion Chatziioannou and the staff in the small-animal imaging facility at Crump Institute for Molecular Imaging for performing the mouse PET imaging, Dr. Michael Kreissl for taking serial blood samples, and Dr. N. Satyamurthy and his staff in the UCLA medical cyclotron facility for producing the tracer FLT used in this study. This work was partly supported by DOE cooperative agreement DE-FC03-02ER63420, and by NIH grants RO1-EB001943 and P50-CA086306.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, M.H., Huang, S.C., Liao, Y.P. et al. FLT-PET Imaging of Radiation Responses in Murine Tumors. Mol Imaging Biol 10, 325–334 (2008). https://doi.org/10.1007/s11307-008-0158-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0158-z