Abstract
Background
The water-perfusable tissue index (PTI) is assumed to differentiate viable myocardium from scar tissue, but histological comparisons in humans are lacking. The present study compares PTI with delayed contrast-enhanced magnetic resonance imaging (DCE-MRI), a validated marker of fibrotic tissue, in patients with ischemic left ventricular (LV) dysfunction. In addition, the optimal PTI threshold for detection of myocardial viability was defined when DCE-MRI was taken as a reference.
Materials
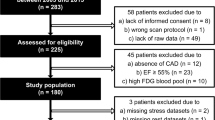
Twenty patients with ischemic LV dysfunction were studied with positron emission tomography, using oxygen-15-labeled water and carbon monoxide as tracers, and DCE-MRI.
Results
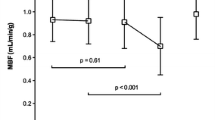
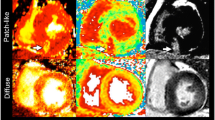
Of the 200 analyzed segments, 112 demonstrated DCE and were subsequently divided in three subgroups according to the severity of enhancement. PTI was 1.04 ± 0.21 in control segments and gradually decreased with increasing extent of DCE to 0.77 ± 0.31 for segments with transmural enhancement (p < 0.001). However, PTI progressively underestimated infarct size with increasing quantities of scar tissue (r = 0.61, p < 0.01). A PTI cutoff value of 0.89 yielded the best diagnostic accuracy for detection of myocardial viability with sensitivity and specificity values of 75 and 77%, respectively.
Conclusions
PTI is inversely related to the extent of scar tissue estimated by DCE-MRI in patients with chronic ischemic heart disease and LV dysfunction. However, with increasing quantities of scar tissue, PTI overestimates the extent of residual viable tissue. A PTI threshold of 0.89 yields the best diagnostic accuracy for viability detection.





Similar content being viewed by others
References
Yamamoto Y, de Silva R, Rhodes CG, Araujo LI, Iida H, Rechavia E, Nihoyannopoulos P, Hackett D, Galassi AR, Taylor CJ (1992) A new strategy for the assessment of viable myocardium and regional myocardial blood flow using 15O-water and dynamic positron emission tomography. Circulation 86:167–178
de Silva R, Yamamoto Y, Rhodes CG, Iida H, Nihoyannopoulos P, Davies GJ, Lammertsma AA, Jones T, Maseri A (1992) Preoperative prediction of the outcome of coronary revascularization using positron emission tomography. Circulation 86:1738–1742
Gerber BL, Melin JA, Bol A, Labar D, Cogneau M, Michel C, Vanoverschelde JL (1998) Nitrogen-13-ammonia and oxygen-15-water estimates of absolute myocardial perfusion in left ventricular ischemic dysfunction. J Nucl Med 39:1655–1662
Itoh H, Namura M, Seki H, Asai T, Tsuchiya T, Uenishi H, Fujii H, Fujita S, Tanabe Y, Ito J, Shimizu M, Mabuchi H (2002) Perfusable tissue index obtained by positron emission tomography as a marker of myocardial viability in patients with ischemic ventricular dysfunction. Circ J 66:341–344
Knaapen P, Boellaard R, Gotte MJ, van der Weerdt AP, Visser CA, Lammertsma AA, Visser FC (2003) The perfusable tissue index: a marker of myocardial viability. J Nucl Cardiol 10:684–691
Iida H, Tamura Y, Kitamura K, Bloomfield PM, Eberl S, Ono Y (2000) Histochemical correlates of (15)O-water-perfusable tissue fraction in experimental canine studies of old myocardial infarction. J Nucl Med 41:1737–1745
Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM (2003) Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 361:374–379
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM (1999) Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100:1992–2002
Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JA, Mohan V, Becker LC, Zerhouni EA (1995) Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation 92:1902–1910
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343:1445–1453
Wellnhofer E, Olariu A, Klein C, Grafe M, Wahl A, Fleck E, Nagel E (2004) Magnetic resonance low-dose dobutamine test is superior to SCAR quantification for the prediction of functional recovery. Circulation 109:2172–2174
Knaapen P, Boellaard R, Gotte MJ, Dijkmans PA, van Campen LM, de Cock CC, Luurtsema G, Visser CA, Lammertsma AA, Visser FC (2004) Perfusable tissue index as a potential marker of fibrosis in patients with idiopathic dilated cardiomyopathy. J Nucl Med 45:1299–1304
Iida H, Rhodes CG, de Silva R, Yamamoto Y, Araujo LI, Maseri A, Jones T (1991) Myocardial tissue fraction—correction for partial volume effects and measure of tissue viability. J Nucl Med 32:2169–2175
Kuhl HP, Beek AM, van der Weerdt AP, Hofman MB, Visser CA, Lammertsma AA, Heussen N, Visser FC, van Rossum AC (2003) Myocardial viability in chronic ischemic heart disease: comparison of contrast-enhanced magnetic resonance imaging with (18)F-fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 41:1341–1348
Hermansen F, Rosen SD, Fath-Ordoubadi F, Kooner JS, Clark JC, Camici PG, Lammertsma AA (1998) Measurement of myocardial blood flow with oxygen-15 labelled water: comparison of different administration protocols. Eur J Nucl Med 25:751–759
Bondarenko O, Beek AM, Hofman MB, Kuhl HP, Twisk JW, van Dockum WG, Visser CA, van Rossum AC (2004) Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 7:481–485
Herrero P, Staudenherz A, Walsh JF, Gropler RJ, Bergmann SR (1995) Heterogeneity of myocardial perfusion provides the physiological basis of perfusable tissue index. J Nucl Med 36:320–327
Iida H, Kanno I, Takahashi A, Miura S, Murakami M, Takahashi K, Ono Y, Shishido F, Inugami A, Tomura N (1988) Measurement of absolute myocardial blood flow with H215O and dynamic positron–emission tomography. Strategy for quantification in relation to the partial-volume effect. Circulation 78:104–115
Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ (2004) The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 43:2260–2264
Alhaddad IA, Kloner RA, Hakim I, Garno JL, Brown EJ (1996) Benefits of late coronary artery reperfusion on infarct expansion progressively diminish over time: relation to viable islets of myocytes within the scar. Am Heart J 131:451–457
Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG (2004) Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 109:2411–2416
Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, Neubauer S (2004) Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation 110:1535–1541
Bax JJ, Fath-Ordoubadi F, Boersma E, Wijns W, Camici PG (2002) Accuracy of PET in predicting functional recovery after revascularisation in patients with chronic ischaemic dysfunction: head-to-head comparison between blood flow, glucose utilisation and water-perfusable tissue fraction. Eur J Nucl Med Mol Imaging 29:721–727
Marinho NV, Keogh BE, Costa DC, Lammerstma AA, Ell PJ, Camici PG (1996) Pathophysiology of chronic left ventricular dysfunction. New insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation 93:737–744
Camici PG, Rimoldi OE (2003) Myocardial blood flow in patients with hibernating myocardium. Cardiovasc Res 57:302–311
Acknowledgment
Olga Bondarenko is supported by the Netherlands Heart Foundation (grant 2001B158).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knaapen, P., Bondarenko, O., Beek, A.M. et al. Impact of Scar on Water-Perfusable Tissue Index in Chronic Ischemic Heart Disease. Mol Imaging Biol 8, 245–251 (2006). https://doi.org/10.1007/s11307-006-0044-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-006-0044-5