Abstract
Purpose
Imaging purine receptors and adenylate biodistribution in vivo may be of clinical importance not only for the investigation of normal adenylate metabolism but also in pathological conditions where adenylate uptake and/or release from certain tissues and organs may be altered, such as some types of cancer. In order to develop a tracer for positron emission tomography (PET) that would not be subject to loss of its radioisotope, adenosine 5′-monophosphate (AMP) was intrinsically labeled at the C-8 position with carbon-11.
Procedures
[11C]AMP was synthesized by reacting 5-amino-1-β-d-ribofuranosylimidazole-4-carboxamidine-5′-phosphate with [11C]formaldehyde. The metabolism of [11C]AMP in human blood was determined in vitro both in the presence and absence of dipyridamole. The ex vivo biodistribution of [11C]AMP and its in vivo dosimetry were determined in normal mice. The effect of dipyridamole on the distribution of [11C]AMP in mice was also determined.
Results
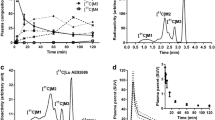
[11C]AMP was reliably synthesized in 34 minutes (n = 7) with an average radiochemical yield of 2.4% and an average specific activity of 90.10 GBq/μmol (2435 mCi/μmol) at end of synthesis. In normal mice, the highest uptake of [11C]AMP was in the lungs, blood, and heart. The ex vivo mouse experiments showed that the uptake of 11C radiotracer in the lungs at 60 minutes postinjection was significantly lower for dipyridamole-treated animals than controls. Dosimetry showed that the critical organs for radiation dose burden are kidneys and bladder.
Conclusions
Treatment with dipyridamole blocked the red blood cell uptake of extracellular adenosine and therefore its subsequent intracellular conversion to ATP. The biodistribution studies indicate that the tracer has substantial accumulation in the kidneys, lungs, heart, and blood. [11C]AMP is promising as a PET-imaging agent to trace adenylate biology in vivo.






Similar content being viewed by others
References
G Manfredi L Yang CD Gajewski M Mattiazzi (2002) ArticleTitleMeasurements of ATP in mammalian cells Methods 26 317–326 Occurrence Handle10.1016/S1046-2023(02)00037-3 Occurrence Handle1:CAS:528:DC%2BD38Xks1Ghurk%3D Occurrence Handle12054922
FI Ataullakhanov VM Vitvitsky (2002) ArticleTitleWhat determines the intracellular ATP concentration Biosci Rep 22 501–511 Occurrence Handle10.1023/A:1022069718709 Occurrence Handle1:CAS:528:DC%2BD3sXlsleltA%3D%3D Occurrence Handle12635847
G Ronquist A Waldenstrom (2003) ArticleTitleImbalance of plasma membrane ion leak and pump relationship as a new aetiological basis of certain disease states J Intern Med 254 517–526 Occurrence Handle10.1111/j.1365-2796.2003.01235.x Occurrence Handle1:CAS:528:DC%2BD2cXjvVGhuw%3D%3D Occurrence Handle14641792
CG Kim DJ Yang EE Kim et al. (1996) ArticleTitleAssessment of tumor cell proliferation using [18F] fluorodeoxyadenosine and [18F] fluoroethyluracil J Pharm Sci 85 339–344 Occurrence Handle10.1021/js950402i Occurrence Handle1:CAS:528:DyaK28XpsFKntQ%3D%3D Occurrence Handle8699341
Sz Lehel G Horváth I Boros P Mikecz T Márián L Trón (2000) ArticleTitleSynthesis of 5′-deoxy-5′-[18F] fluoroadenosine by radiofluorination of 5′-deoxy-5′-haloadenosine derivatives J Radioanal Nucl Chem 245 399–401 Occurrence Handle10.1023/A:1006791211919 Occurrence Handle1:CAS:528:DC%2BD3cXmtFCms74%3D
Sz Lehel G Horváth I Boros et al. (2000) ArticleTitleSynthesis of 5′-N-(2-[18F] fluoroethyl)-carboamidoadenosine: A promising tracer for investigation of adenosine receptor system by PET technique J Label Compd Radiopharm 43 807–815 Occurrence Handle10.1002/1099-1344(200007)43:8<807::AID-JLCR365>3.0.CO;2-K Occurrence Handle1:CAS:528:DC%2BD3cXksF2mtbo%3D
T Márián Sz Lehel Z Lengyel et al. (2002) ArticleTitle[18F]-FNECA serves as a suitable radioligand for PET investigation of purinergic receptor expression Orv Hetil 143 1319–1322 Occurrence Handle12077928
MM Alauddin JD Fissekis PS Conti (2003) ArticleTitleSynthesis of [18F]-labeled adenosine analogs as potential PET imaging agents J Label Compd Radiopharm 46 805–814 Occurrence Handle10.1002/jlcr.712 Occurrence Handle1:CAS:528:DC%2BD3sXntFeisLY%3D
L Martarello C Schaffrath H Deng AD Gee A Lockhart D O’Hagan (2003) ArticleTitleThe first enzymatic method for C–18F bond formation: The synthesis of 5′-[18F]-fluoro-5′-deoxyadenosine for imaging with PET J Label Compd Radiopharm 46 1181–1189 Occurrence Handle10.1002/jlcr.779 Occurrence Handle1:CAS:528:DC%2BD3sXhtVSgsL7E
RB Meyer SuffixJr CG Wong (1981) ArticleTitleA convenient synthesis of carbon labelled adenine nucleotides: Adenosine-2-13C 5′-phosphate J Label Compd Radiopharm 18 1119–1122 Occurrence Handle1:CAS:528:DyaL38XhtVClt7k%3D
MW Nader SK Zeisler A Theobald F Oberdorfer (1998) ArticleTitleLow temperature synthesis of no-carrier-added [11C] formaldehyde with metal hydrides and preparation of [1-11C] 1,2,3,4-tetrahydro-β-carboline derivatives Appl Radiat Isot 49 1599–1603 Occurrence Handle10.1016/S0969-8043(97)10156-7 Occurrence Handle1:CAS:528:DyaK1cXls1Ogtrg%3D
D Roeda C Crouzel (2001) ArticleTitle[11C] Formaldehyde revisited: Considerable concurrent [11C] formic acid formation in the low-temperature conversion of [11C] carbon dioxide into [11C] formaldehyde Appl Radiat Isot 54 935–939 Occurrence Handle10.1016/S0969-8043(00)00352-3 Occurrence Handle1:CAS:528:DC%2BD3MXhtlSlt70%3D Occurrence Handle11300407
WB Mathews HT Ravert U Scheffel et al. (2002) ArticleTitle[11C] 5′-adenosine monophosphate ([11C] AMP): A new radioligand for positron emission tomographic imaging of cancer J Nucl Med 43 362P
Abraham EH (2003) Adenylate diagnostics LLC. Labeled adenosine for use in positron emission tomography. Patent Application 3418.1000-000, 22 May
M Kopff I Zakrzewska J Klem B Zachara (1986) ArticleTitleEffect of dipyridamole on adenosine incorporation into hypoxanthine nucleotides of fresh human red cells Haematologia 19 89–94 Occurrence Handle1:CAS:528:DyaL28Xls12ltbs%3D Occurrence Handle3758842
J Spychala (2000) ArticleTitleTumor-promoting functions of adenosine Pharmacol Ther 87 161–173 Occurrence Handle10.1016/S0163-7258(00)00053-X Occurrence Handle1:CAS:528:DC%2BD3cXmslOjtbo%3D Occurrence Handle11007998
Acknowledgments
The initial theory for the chemical synthesis and use of [11C]AMP was proposed by Dr. Abraham. The authors wish to thank Mr. Robert C. Smoot for his assistance with cyclotron operation and radiosynthesis. This work was supported by NIH grant number CA52880.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathews, W.B., Nakamoto, Y., Abraham, E.H. et al. Synthesis and Biodistribution of [11C]Adenosine 5′-Monophosphate ([11C]AMP). Mol Imaging Biol 7, 203–208 (2005). https://doi.org/10.1007/s11307-005-4118-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-005-4118-6