Abstract
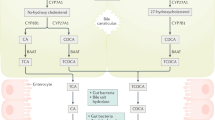
Specific transporters expressed in the liver and the intestine, play a critical role in driving the enterohepatic circulation of bile acids. By preserving a circulating pool of bile acids, an important factor influencing bile flow, these transporters are involved in maintaining bile acid and cholesterol homeostasis. Enterohepatic circulation of bile acids is fundamentally composed of two major processes: secretion from the liver and absorption from the intestine. In the hepatocytes, the vectorial transport of bile acids from blood to bile is ensured by Na+ taurocholate co-transporting peptide (NTCP) and organic anion transport polypeptides (OATPs). After binding to a cytosolic bile acid binding protein, bile acids are secreted into the canaliculus via ATP-dependent bile salt excretory pump (BSEP) and multi drug resistant proteins (MRPs). Bile acids are then delivered to the intestinal lumen through bile ducts where they emulsify dietary lipids and cholesterol to facilitate their absorption. Intestinal epithelial cells reabsorb the majority of the secreted bile acids through the apical sodium dependent bile acid transporter (ASBT) and sodium independent organic anion transporting peptide (OATPs). Cytosolic ileal bile acid binding protein (IBABP) mediates the transcellular movement of bile acids to the basolateral membrane across which they exit the cells via organic solute transporters (OST). An essential role of bile acid transporters is evident from the pathology associated with their genetic disruption or dysregulation of their function. Malfunctioning of hepatic and intestinal bile acid transporters is implicated in the pathophysiology of cholestatic liver disease and the depletion of circulating pool of bile acids, respectively. Extensive efforts have been recently made to enhance our understanding of the structure, function and regulation of the bile acid transporters and exploring new potential therapeutics to treat bile acid or cholesterol related diseases. This review will highlight current knowledge about structure, function and molecular characterization of bile acid transporters and discuss the implications of their defects in various hepatic and intestinal disorders.



Similar content being viewed by others
References
A. F. Hofmann. Biliary secretion and excretion: the hepatobiliary component of the enterohepatic circulation of bile acids. Raven Press, 1994.
A. F. Hofmann. Bile acids: the good, the bad, and the ugly. News Physiol. Sci. 14:24–29 (1999).
A. F. Hofmann. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 159:2647–2658 (1999).
G. A. Kullak-Ublick, B. Stieger, and P. J. Meier. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126:322–342 (2004).
P. J. Meier and B. Stieger. Bile salt transporters. Annu. Rev. Physiol. 64:635–661 (2002).
M. Trauner and J. L. Boyer. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83:633–671 (2003).
S. M. Houten, M. Watanabe, and J. Auwerx. Endocrine functions of bile acids. EMBO J. 25:1419–1425 (2006).
A. Chawla, E. Saez, and R. M. Evans. Don’t know much bile-ology. Cell 103:1–4 (2000).
D. W. Russell. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72:137–174 (2003).
R. N. Redinger. The role of the enterohepatic circulation of bile salts and nuclear hormone receptors in the regulation of cholesterol homeostasis: Bile salts as ligands for nuclear hormone receptors. Can. J. Gastroenterol. 17:265–271 (2003).
R. N. Redinger. Nuclear receptors in cholesterol catabolism: molecular biology of the enterohepatic circulation of bile salts and its role in cholesterol homeostasis. J. Lab. Clin. Med. 142:7–20 (2003).
R. N. Redinger. The coming of age of our understanding of the enterohepatic circulation of bile salts. Am. J. Surg. 185:168–172 (2003).
G. D. Potter. Bile acid diarrhea. Dig. Dis. 16:118–124 (1998).
S. Lowes and N. L. Simmons. Human intestinal cell monolayers are preferentially sensitive to disruption of barrier function from basolateral exposure to cholic acid: correlation with membrane transport and transepithelial secretion. Pflugers Arch. 443:265–273 (2001).
S. Lechner, U. Muller-Ladner, K. Schlottmann, B. Jung, M. McClelland, J. Ruschoff, J. Welsh, J. Scholmerich, and F. Kullmann. Bile acids mimic oxidative stress induced upregulation of thioredoxin reductase in colon cancer cell lines. Carcinogenesis 23:1281–1288 (2002).
H. Bernstein, C. Bernstein, C. M. Payne, K. Dvorakova, and H. Garewal. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 589:47–65 (2005).
W. A. Alrefai, S. Saksena, S. Tyagi, R. K. Gill, K. Ramaswamy, and P. K. Dudeja. Taurodeoxycholic bile acid inhibits chloride uptake in Caco2 cells. Dig. Dis. Sci. In press:(2007).
M. S. Anwer. Transhepatic solute transport and bile formation. Adv. Vet. Sci. Comp. Med. 37:1–29 (1993).
R. J. Bahar and A. Stolz. Bile acid transport. Gastroenterol. Clin. North Am. 28:27–58 (1999).
G. Zollner and M. Trauner. Molecular mechanisms of cholestasis. Wien. Med. Wochenschr. 156:380–385 (2006).
J. J. Eloranta, P. J. Meier, and G. A. Kullak-Ublick. Coordinate transcriptional regulation of transport and metabolism. Methods Enzymol. 400:511–530 (2005).
C. Pauli-Magnus and P. J. Meier. Hepatobiliary transporters and drug-induced cholestasis. Hepatology 44:778–87 (2006).
G. A. Kullak-Ublick, J. Glasa, C. Boker, M. Oswald, U. Grutzner, B. Hagenbuch, B. Stieger, P. J. Meier, U. Beuers, W. Kramer, G. Wess, and G. Paumgartner. Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology 113:1295–1305 (1997).
R. P. Oude Elferink, C. C. Paulusma, and A. K. Groen. Hepatocanalicular transport defects: pathophysiologic mechanisms of rare diseases. Gastroenterology 130:908–925 (2006).
P. J. Meier. Molecular mechanisms of hepatic bile salt transport from sinusoidal blood into bile. Am. J. Physiol. 269:G801–G812 (1995).
L. B. Agellon and E. C. Torchia. Intracellular transport of bile acids. Biochim. Biophys. Acta 1486:198–209 (2000).
T. Horie, T. Mizuma, S. Kasai, and S. Awazu. Conformational change in plasma albumin due to interaction with isolated rat hepatocyte. Am. J. Physiol. 254:G465–G470 (1988).
G. M. Groothuis, M. J. Hardonk, K. P. Keulemans, P. Nieuwenhuis, and D. K. Meijer. Autoradiographic and kinetic demonstration of acinar heterogeneity of taurocholate transport. Am. J. Physiol. 243:G455–G462 (1982).
A. L. Jones, G. T. Hradek, R. H. Renston, K. Y. Wong, G. Karlaganis, and G. Paumgartner. Autoradiographic evidence for hepatic lobular concentration gradient of bile acid derivative. Am. J. Physiol. 238:G233–G237 (1980).
T. J. Layden and J. L. Boyer. Influence of bile acids on bile canalicular membrane morphology and the lobular gradient in canalicular size. Lab. Invest. 39:110–119 (1978).
M. S. Anwerand D. Hegner. Effect of Na on bile acid uptake by isolated rat hepatocytes. Evidence for a heterogeneous system. Hoppe-Seylers Z. Physiol. Chem. 359:181–192 (1978).
R. W. Van Dyke, J. E. Stephens, and B. F. Scharschmidt. Bile acid transport in cultured rat hepatocytes. Am. J. Physiol. 243:G484–G492 (1982).
C. E. Bear, J. S. Davison, and E. A. Shaffer. Sodium-dependent taurocholate uptake by isolated rat hepatocytes occurs through an electrogenic mechanism. Biochim. Biophys. Acta 903:388–394 (1987).
S. A. Weinman, M. W. Carruth, and P. A. Dawson. Bile acid uptake via the human apical sodium-bile acid cotransporter is electrogenic. J. Biol. Chem. 273:34691–34695 (1998).
B. Hagenbuch and P. Dawson. The sodium bile salt cotransport family SLC10. Pflugers Arch. 447:566–570 (2004).
J. Geyer, T. Wilke, and E. Petzinger. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn-Schmiedeberg’s Arch. Pharmacol. 372:413–431 (2006).
M. Ananthanarayanan, O. C. Ng, J. L. Boyer, and F. J. Suchy. Characterization of cloned rat liver Na(+)-bile acid cotransporter using peptide and fusion protein antibodies. Am. J. Physiol. 267:G637–G643 (1994).
J. Y. Kim, K. H. Kim, J. A. Lee, W. Namkung, A. Q. Sun, M. Ananthanarayanan, F. J. Suchy, D. M. Shin, S. Muallem, and M. G. Lee. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology 122:1941–1953 (2002).
S. J. Rippin, B. Hagenbuch, P. J. Meier, and B. Stieger. Cholestatic expression pattern of sinusoidal and canalicular organic anion transport systems in primary cultured rat hepatocytes. Hepatology 33:776–782 (2001).
D. Liang, B. Hagenbuch, P. J. Meier, and B. Stieger. Parallel decrease of Na(+)-taurocholate cotransport and its encoding mRNA in primary cultures of rat hepatocytes. Hepatology 18:1162–1166 (1993).
B. Hagenbuch, B. F. Scharschmidt, and P. J. Meier. Effect of antisense oligonucleotides on the expression of hepatocellular bile acid and organic anion uptake systems in Xenopus laevis oocytes. Biochem. J. 316(Pt 3):901–904 (1996).
B. Hagenbuch and P. J. Meier. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J. Clin. Invest. 93:1326–1331 (1994).
R. M. Green, M. Ananthanarayanan, F. J. Suchy, and D. R. Beier. Genetic mapping of the Na(+)-taurocholate cotransporting polypeptide to mouse chromosome 12. Mamm. Genome 9:598 (1998).
M. A. Cohn, D. J. Rounds, S. J. Karpen, M. Ananthanarayanan, and F. J. Suchy. Assignment of a rat liver Na+/bile acid cotransporter gene to chromosome 6q24. Mamm. Genome 6:60 (1995).
V. Cattori, U. Eckhardt, and B. Hagenbuch. Molecular cloning and functional characterization of two alternatively spliced Ntcp isoforms from mouse liver1. Biochim. Biophys. Acta 1445:154–159 (1999).
S. Hallen, O. Mareninova, M. Branden, and G. Sachs. Organization of the membrane domain of the human liver sodium/bile acid cotransporter. Biochemistry 41:7253–7266 (2002).
O. Mareninova, J. M. Shin, O. Vagin, S. Turdikulova, S. Hallen, and G. Sachs. Topography of the membrane domain of the liver Na+-dependent bile acid transporter. Biochemistry 44:13702–13712 (2005).
A. Schroeder, U. Eckhardt, B. Stieger, R. Tynes, C. D. Schteingart, A. F. Hofmann, P. J. Meier, and B. Hagenbuch. Substrate specificity of the rat liver Na(+)-bile salt cotransporter in Xenopus laevis oocytes and in CHO cells. Am. J. Physiol. 274:G370–G375 (1998).
S. Hata, P. Wang, N. Eftychiou, M. Ananthanarayanan, A. Batta, G. Salen, K. S. Pang, and A. W. Wolkoff. Substrate specificities of rat oatp1 and ntcp: implications for hepatic organic anion uptake. Am. J. Physiol.: Gastrointest. Liver Physiol. 285:G829–G839 (2003).
A. L. Craddock, M. W. Love, R. W. Daniel, L. C. Kirby, H. C. Walters, M. H. Wong, and P. A. Dawson. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am. J. Physiol. 274:G157–G169 (1998).
S. Hallen, J. Fryklund, and G. Sachs. Inhibition of the human sodium/bile acid cotransporters by side-specific methanethiosulfonate sulfhydryl reagents: substrate-controlled accessibility of site of inactivation. Biochemistry 39:6743–6750 (2000).
D. Zahner, U. Eckhardt, and E. Petzinger. Transport of taurocholate by mutants of negatively charged amino acids, cysteines, and threonines of the rat liver sodium-dependent taurocholate cotransporting polypeptide Ntcp. Eur. J. Biochem. 270:1117–1127 (2003).
T. Saeki, T. Kuroda, M. Matsumoto, R. Kanamoto, and K. Iwami. Effects of Cys mutation on taurocholic acid transport by mouse ileal and hepatic sodium-dependent bile acid transporters. Biosci. Biotechnol. Biochem. 66:467–470 (2002).
R. H. Ho, B. F. Leake, R. L. Roberts, W. Lee, and R. B. Kim. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J. Biol. Chem. 279:7213–7222 (2004).
M. Arrese, M. Trauner, M. Ananthanarayanan, M. Pizarro, N. Solis, L. Accatino, C. Soroka, J. L. Boyer, S. J. Karpen, J. F. Miquel, and F. J. Suchy. Down-regulation of the Na+/taurocholate cotransporting polypeptide during pregnancy in the rat. J. Hepatol. 38:148–155 (2003).
G. Zollner, P. Fickert, D. Silbert, A. Fuchsbichler, H. U. Marschall, K. Zatloukal, H. Denk, and M. Trauner. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J. Hepatol. 38:717–727 (2003).
G. Zollner, P. Fickert, R. Zenz, A. Fuchsbichler, C. Stumptner, L. Kenner, P. Ferenci, R. E. Stauber, G. J. Krejs, H. Denk, K. Zatloukal, and M. Trauner. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology 33:633–646 (2001).
M. S. Anwer. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology 39:581–590 (2004).
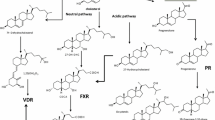
J. Y. Chiang. Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am. J. Physiol. Gastrointest. Liver. Physiol. 284:G349–G356 (2003).
L. A. Denson, E. Sturm, W. Echevarria, T. L. Zimmerman, M. Makishima, D. J. Mangelsdorf, and S. J. Karpen. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 121:140–147 (2001).
D. Jungand G. A. Kullak-Ublick. Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression. Hepatology 37:622–631 (2003).
M. Trauner, M. Arrese, H. Lee, J. L. Boyer, and S. J. Karpen. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J. Clin. Invest. 101:2092–2100 (1998).
L. A. Denson, K. L. Auld, D. S. Schiek, M. H. McClure, D. J. Mangelsdorf, and S. J. Karpen. Interleukin-1beta suppresses retinoid transactivation of two hepatic transporter genes involved in bile formation. J. Biol. Chem. 275:8835–8843 (2000).
D. Li, T. L. Zimmerman, S. Thevananther, H. Y. Lee, J. M. Kurie, and S. J. Karpen. Interleukin-1 beta-mediated suppression of RXR: RAR transactivation of the Ntcp promoter is JNK-dependent. J. Biol. Chem. 277:31416–31422 (2002).
J. J. Eloranta, D. Jung, and G. A. Kullak-Ublick. The human Na+-taurocholate cotransporting polypeptide gene is activated by glucocorticoid receptor and peroxisome proliferator-activated receptor-gamma coactivator-1alpha, and suppressed by bile acids via a small heterodimer partner-dependent mechanism. Mol. Endocrinol. 20:65–79 (2006).
T. C. Ganguly, M. L. O’Brien, S. J. Karpen, J. F. Hyde, F. J. Suchy, and M. Vore. Regulation of the rat liver sodium-dependent bile acid cotransporter gene by prolactin. Mediation of transcriptional activation by Stat5. J. Clin. Invest. 99:2906–2914 (1997).
M. S. Anwer, H. Gillin, S. Mukhopadhyay, N. Balasubramaniyan, F. J. Suchy, and M. Ananthanarayanan. Dephosphorylation of Ser-226 facilitates plasma membrane retention of Ntcp. J. Biol. Chem. 280:33687–33692 (2005).
Q. Zhu, P. von Dippe, W. Xing, and D. Levy. Membrane topology and cell surface targeting of microsomal epoxide hydrolase. Evidence for multiple topological orientations. J. Biol. Chem. 274:27898–27904 (1999).
P. von Dippe, M. Amoui, R. H. Stellwagen, and D. Levy. The functional expression of sodium-dependent bile acid transport in Madin–Darby canine kidney cells transfected with the cDNA for microsomal epoxide hydrolase. J. Biol. Chem. 271:18176–18180 (1996).
M. Miyata, G. Kudo, Y. H. Lee, T. J. Yang, H. V. Gelboin, P. Fernandez-Salguero, S. Kimura, and F. J. Gonzalez. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. J. Biol. Chem. 274:23963–23968 (1999).
Q. S. Zhu, W. Xing, B. Qian, P. von Dippe, B. L. Shneider, V. L. Fox, and D. Levy. Inhibition of human m-epoxide hydrolase gene expression in a case of hypercholanemia. Biochim. Biophys. Acta 1638:208–216 (2003).
B. Zimmerli, J. Valantinas, and P. J. Meier. Multispecificity of Na+-dependent taurocholate uptake in basolateral (sinusoidal) rat liver plasma membrane vesicles. J. Pharmacol. Exp. Ther. 250:301–308 (1989).
B. Hagenbuch and P. J. Meier. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta 1609:1–18 (2003).
B. Hagenbuch and P. J. Meier. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 447:653–665 (2004).
G. A. Kullak-Ublick, B. Hagenbuch, B. Stieger, C. D. Schteingart, A. F. Hofmann, A. W. Wolkoff, and P. J. Meier. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology 109:1274–1282 (1995).
I. Tamai, J. Nezu, H. Uchino, Y. Sai, A. Oku, M. Shimane, and A. Tsuji. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 273:251–260 (2000).
T. K. Lee, C. L. Hammond, and N. Ballatori. Intracellular glutathione regulates taurocholate transport in HepG2 cells. Toxicol. Appl. Pharmacol. 174:207–215 (2001).
M. Kakyo, H. Sakagami, T. Nishio, D. Nakai, R. Nakagomi, T. Tokui, T. Naitoh, S. Matsuno, T. Abe, and H. Yawo. Immunohistochemical distribution and functional characterization of an organic anion transporting polypeptide 2 (oatp2). FEBS Lett. 445:343–6 (1999).
C. Reichel, B. Gao, J. Van Montfoort, V. Cattori, C. Rahner, B. Hagenbuch, B. Stieger, T. Kamisako, and P. J. Meier. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology 117:688–695 (1999).
G. A. Kullak-Ublick, M. G. Ismair, B. Stieger, L. Landmann, R. Huber, F. Pizzagalli, K. Fattinger, P. J. Meier, and B. Hagenbuch. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533 (2001).
J. Konig, Y. Cui, A. T. Nies, and D. Keppler. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G156–G164 (2000).
R. G. Tirona, B. F. Leake, G. Merino, and R. B. Kim. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J. Biol. Chem. 276:35669–35675 (2001).
T. Nozawa, M. Nakajima, I. Tamai, K. Noda, J. Nezu, Y. Sai, A. Tsuji, and T. Yokoi. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J. Pharmacol. Exp. Ther. 302:804–813 (2002).
T. Abe, M. Unno, T. Onogawa, T. Tokui, T. N. Kondo, R. Nakagomi, H. Adachi, K. Fujiwara, M. Okabe, T. Suzuki, K. Nunoki, E. Sato, M. Kakyo, T. Nishio, J. Sugita, N. Asano, M. Tanemoto, M. Seki, F. Date, K. Ono, Y. Kondo, K. Shiiba, M. Suzuki, H. Ohtani, T. Shimosegawa, K. Iinuma, H. Nagura, S. Ito, and S. Matsuno. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120:1689–1699 (2001).
J. Konig, Y. Cui, A. T. Nies, and D. Keppler. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J. Biol. Chem. 275:23161–23168 (2000).
D. Q. Shih, M. Bussen, E. Sehayek, M. Ananthanarayanan, B. L. Shneider, F. J. Suchy, S. Shefer, J. S. Bollileni, F. J. Gonzalez, J. L. Breslow, and M. Stoffel. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat. Genet. 27:375–382 (2001).
G. P. Hayhurst, Y. H. Lee, G. Lambert, J. M. Ward, and F. J. Gonzalez. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21:1393–1403 (2001).
F. R. Simon, J. Fortune, M. Iwahashi, S. Bowman, A. Wolkoff, and E. Sutherland. Characterization of the mechanisms involved in the gender differences in hepatic taurocholate uptake. Am. J. Physiol. 276:G556–G565 (1999).
R. Lu, N. Kanai, Y. Bao, A. W. Wolkoff, and V. L. Schuster. Regulation of renal oatp mRNA expression by testosterone. Am. J. Physiol. 270:F332–F337 (1996).
Y. Gotoh, Y. Kato, B. Stieger, P. J. Meier, and Y. Sugiyama. Gender difference in the Oatp1-mediated tubular reabsorption of estradiol 17beta-D-glucuronide in rats. Am. J. Physiol., Endocrinol. Metab. 282:E1245–E1254 (2002).
G. L. Guo, D. R. Johnson, and C. D. Klaassen. Postnatal expression and induction by pregnenolone-16alpha-carbonitrile of the organic anion-transporting polypeptide 2 in rat liver. Drug Metab. Dispos. 30:283–288 (2002).
P. Fickert, G. Zollner, A. Fuchsbichler, C. Stumptner, C. Pojer, R. Zenz, F. Lammert, B. Stieger, P. J. Meier, K. Zatloukal, H. Denk, and M. Trauner. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology 121:170–183 (2001).
M. Oswald, G. A. Kullak-Ublick, G. Paumgartner, and U. Beuers. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver 21:247–253 (2001).
D. Jung, M. Podvinec, U. A. Meyer, D. J. Mangelsdorf, M. Fried, P. J. Meier, and G. A. Kullak-Ublick. Human organic anion transporting polypeptide 8 promoter is transactivated by the farnesoid X receptor/bile acid receptor. Gastroenterology 122:1954–1966 (2002).
G. L. Guo and C. D. Klaassen. Protein kinase C suppresses rat organic anion transporting polypeptide 1- and 2-mediated uptake. J. Pharmacol. Exp. Ther. 299:551–557 (2001).
C. J. Soroka, J. M. Lee, F. Azzaroli, and J. L. Boyer. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 33:783–791 (2001).
M. Rius, A. T. Nies, J. Hummel-Eisenbeiss, G. Jedlitschky, and D. Keppler. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology 38:374–384 (2003).
J. Konig, D. Rost, Y. Cui, and D. Keppler. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology 29:1156–1163 (1999).
H. Zeng, G. Liu, P. A. Rea, and G. D. Kruh. Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res. 60:4779–4784 (2000).
N. Zelcer, K. van de Wetering, R. de Waart, G. L. Scheffer, H. U. Marschall, P. R. Wielinga, A. Kuil, C. Kunne, A. Smith, M. van der Valk, J. Wijnholds, R. O. Elferink, and P. Borst. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J. Hepatol. 44:768–775 (2006).
H. Takikawa, Y. Sugiyama, J. C. Fernandez-Checa, J. Kuhlenkamp, M. Ookhtens, and N. Kaplowitz. Evidence that interference with binding to hepatic cytosol binders can inhibit bile acid excretion in rats. Hepatology 23:1642–1649 (1996).
C. J. Sinal, M. Tohkin, M. Miyata, J. M. Ward, G. Lambert, and F. J. Gonzalez. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744 (2000).
F. J. Suchyand M. Ananthanarayanan. Bile salt excretory pump: biology and pathobiology. J. Pediatr. Gastroenterol. Nutr. 43(Suppl 1):S10–S16 (2006).
M. Arrese and M. Ananthanarayanan. The bile salt export pump: molecular properties, function and regulation. Pflugers Arch. 449:123–131 (2004).
M. V. St-Pierre, G. A. Kullak-Ublick, B. Hagenbuch, and P. J. Meier. Transport of bile acids in hepatic and non-hepatic tissues. J. Exp. Biol. 204:1673–1686 (2001).
R. Wang, M. Salem, I. M. Yousef, B. Tuchweber, P. Lam, S. J. Childs, C. D. Helgason, C. Ackerley, M. J. Phillips, and V. Ling. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc. Natl. Acad. Sci. U. S. A. 98:2011–2016 (2001).
T. Gerloff, B. Stieger, B. Hagenbuch, J. Madon, L. Landmann, J. Roth, A. F. Hofmann, and P. J. Meier. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 273:10046–10050 (1998).
R. M. Green, F. Hoda, and K. L. Ward. Molecular cloning and characterization of the murine bile salt export pump. Gene 241:117–123 (2000).
G. Xu, L. X. Pan, S. K. Erickson, B. M. Forman, B. L. Shneider, M. Ananthanarayanan, X. Li, S. Shefer, N. Balasubramanian, L. Ma, H. Asaoka, S. R. Lear, L. B. Nguyen, I. Dussault, F. J. Suchy, G. S. Tint, and G. Salen. Removal of the bile acid pool upregulates cholesterol 7alpha-hydroxylase by deactivating FXR in rabbits. J. Lipid Res. 43:45–50 (2002).
J. A. Byrne, S. S. Strautnieks, G. Mieli-Vergani, C. F. Higgins, K. J. Linton, and R. J. Thompson. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology 123:1649–1658 (2002).
J. Noe, B. Stieger, and P. J. Meier. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology 123:1659–1666 (2002).
G. Tomer, M. Ananthanarayanan, A. Weymann, N. Balasubramanian, and F. J. Suchy. Differential developmental regulation of rat liver canalicular membrane transporters Bsep and Mrp2. Pediatr. Res. 53:288–294 (2003).
H. Wolters, B. M. Elzinga, J. F. Baller, R. Boverhof, M. Schwarz, B. Stieger, H. J. Verkade, and F. Kuipers. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J. Hepatol. 37:556–563 (2002).
M. Ananthanarayanan, S. Li, N. Balasubramaniyan, F. J. Suchy, and M. J. Walsh. Ligand-dependent activation of the farnesoid X-receptor directs arginine methylation of histone H3 by CARM1. J. Biol. Chem. 279:54348–54357 (2004).
G. Rizzo, B. Renga, E. Antonelli, D. Passeri, R. Pellicciari, and S. Fiorucci. The methyl transferase PRMT1 functions as co-activator of farnesoid X receptor (FXR)/9-cis retinoid X receptor and regulates transcription of FXR responsive genes. Mol. Pharmacol. 68:551–558 (2005).
F. A. Crocenzi, A. D. Mottino, E. J. Sanchez Pozzi, J. M. Pellegrino, E. A. Rodriguez Garay, P. Milkiewicz, M. Vore, R. Coleman, and M. G. Roma. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut 52:1170–1177 (2003).
F. A. Crocenzi, E. J. Sanchez Pozzi, J. M. Pellegrino, E. A. Rodriguez Garay, A. D. Mottino, and M. G. Roma. Preventive effect of silymarin against taurolithocholate-induced cholestasis in the rat. Biochem. Pharmacol. 66:355–364 (2003).
P. L. Jansen, S. S. Strautnieks, E. Jacquemin, M. Hadchouel, E. M. Sokal, G. J. Hooiveld, J. H. Koning, A. De Jager-Krikken, F. Kuipers, F. Stellaard, C. M. Bijleveld, A. Gouw, H. Van Goor, R. J. Thompson, and M. Muller. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117:1370–1379 (1999).
L. Wang, C. J. Soroka, and J. L. Boyer. The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J. Clin. Invest. 110:965–972 (2002).
R. Thompson and S. Strautnieks. BSEP: function and role in progressive familial intrahepatic cholestasis. Semin. Liver Dis. 21:545–550 (2001).
L. N. Bull, M. J. van Eijk, L. Pawlikowska, J. A. DeYoung, J. A. Juijn, M. Liao, L. W. Klomp, N. Lomri, R. Berger, B. F. Scharschmidt, A. S. Knisely, R. H. Houwen, and N. B. Freimer. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat. Genet. 18:219–224 (1998).
P. L. Jansen and E. Sturm. Genetic cholestasis, causes and consequences for hepatobiliary transport. Liver Int. 23:315–322 (2003).
P. Ujhazy, D. Ortiz, S. Misra, S. Li, J. Moseley, H. Jones, and I. M. Arias. Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology 34:768–775 (2001).
F. Chen, M. Ananthanarayanan, S. Emre, E. Neimark, L. N. Bull, A. S. Knisely, S. S. Strautnieks, R. J. Thompson, M. S. Magid, R. Gordon, N. Balasubramanian, F. J. Suchy, and B. L. Shneider. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology 126:756–764 (2004).
M. Schmitt, R. Kubitz, S. Lizun, M. Wettstein, and D. Haussinger. Regulation of the dynamic localization of the rat Bsep gene-encoded bile salt export pump by anisoosmolarity. Hepatology 33:509–518 (2001).
H. Kipp, N. Pichetshote, and I. M. Arias. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J. Biol. Chem. 276:7218–7224 (2001).
R. Kubitz, G. Sutfels, T. Kuhlkamp, R. Kolling, and D. Haussinger. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology 126:541–553 (2004).
J. Noe, B. Hagenbuch, P. J. Meier, and M. V. St-Pierre. Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology 33:1223–1231 (2001).
M. A. van Kuijck, M. Kool, G. F. Merkx, A. Geurts van Kessel, R. J. Bindels, P. M. Deen, and C. H. van Os. Assignment of the canalicular multispecific organic anion transporter gene (CMOAT) to human chromosome 10q24 and mouse chromosome 19D2 by fluorescent in situ hybridization. Cytogenet. Cell Genet. 77:285–287 (1997).
F. Lammert, D. E. Cohen, B. Paigen, M. C. Carey, and D. R. Beier. The gene encoding the multispecific organic anion transporter (Cmoat) of the hepatocyte canalicular membrane maps to mouse chromosome 19. Mamm. Genome. 9:87–88 (1998).
P. M. Gerkand M. Vore. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J. Pharmacol. Exp. Ther. 302:407–415 (2002).
M. Niemi, K. A. Arnold, J. T. Backman, M. K. Pasanen, U. Godtel-Armbrust, L. Wojnowski, U. M. Zanger, P. J. Neuvonen, M. Eichelbaum, K. T. Kivisto, and T. Lang. Association of genetic polymorphism in ABCC2 with hepatic multidrug resistance-associated protein 2 expression and pravastatin pharmacokinetics. Pharmacogenet. Genomics. 16:801–808 (2006).
A. Lindahl, A. Sjoberg, U. Bredberg, H. Toreson, A. L. Ungell, and H. Lennernas. Regional intestinal absorption and biliary excretion of fluvastatin in the rat: possible involvement of mrp2. Mol. Pharm. 1:347–356 (2004).
K. Ito, H. Suzuki, and Y. Sugiyama. Single amino acid substitution of rat MRP2 results in acquired transport activity for taurocholate. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G1034–G1043 (2001).
R. O. Elferink and A. K. Groen. Genetic defects in hepatobiliary transport. Biochim. Biophys. Acta 1586:129–145 (2002).
J. A. Barnard and F. K. Ghishan. Taurocholate transport by human ileal brush border membrane vesicles. Gastroenterology 93:925–933 (1987).
S. L. Weinberg, G. Burckhardt, and F. A. Wilson. Taurocholate transport by rat intestinal basolateral membrane vesicles. Evidence for the presence of an anion exchange transport system. J. Clin. Invest. 78:44–50 (1986).
W. A. Alrefai, Z. Sarwar, S. Tyagi, S. Saksena, P. K. Dudeja, and R. K. Gill. Cholesterol modulates human intestinal sodium-dependent bile acid transporter. Am. J. Physiol. Gastrointest. Liver Physiol. 288:G978–G985 (2005).
P. A. Dawson, M. Hubbert, J. Haywood, A. L. Craddock, N. Zerangue, W. V. Christian, and N. Ballatori. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 280:6960–6968 (2005).
A. Balakrishnan, D. J. Sussman, and J. E. Polli. Development of stably transfected monolayer overexpressing the human apical sodium-dependent bile acid transporter (hASBT). Pharm. Res. 22:1269–1280 (2005).
C. McClintock and Y. F. Shiau. Jejunum is more important than terminal ileum for taurocholate absorption in rats. Am. J. Physiol. 244:G507–G514 (1983).
M. H. Wong, P. Oelkers, A. L. Craddock, and P. A. Dawson. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J. Biol. Chem. 269:1340–1347 (1994).
M. H. Wong, P. Oelkers, and P. A. Dawson. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J. Biol. Chem. 270:27228–27234 (1995).
B. L. Shneider, P. A. Dawson, D. M. Christie, W. Hardikar, M. H. Wong, and F. J. Suchy. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J. Clin. Invest. 95:745–754 (1995).
W. Kramer, S. Stengelin, K. H. Baringhaus, A. Enhsen, H. Heuer, W. Becker, D. Corsiero, F. Girbig, R. Noll, and C. Weyland. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J. Lipid Res. 40:1604–1617 (1999).
T. Saeki, K. Matoba, H. Furukawa, K. Kirifuji, R. Kanamoto, and K. Iwami. Characterization, cDNA cloning, and functional expression of mouse ileal sodium-dependent bile acid transporter. J. Biochem. (Tokyo) 125:846–851 (1999).
M. H. Wong, P. N. Rao, M. J. Pettenati, and P. A. Dawson. Localization of the ileal sodium-bile acid cotransporter gene (SLC10A2) to human chromosome 13q33. Genomics 33:538–540 (1996).
B. L. Shneider, K. D. Setchell, and M. W. Crossman. Fetal and neonatal expression of the apical sodium-dependent bile acid transporter in the rat ileum and kidney. Pediatr. Res. 42:189–194 (1997).
S. Hallen, M. Branden, P. A. Dawson, and G. Sachs. Membrane insertion scanning of the human ileal sodium/bile acid co-transporter. Biochemistry 38:11379–11388 (1999).
A. Banerjee and P. W. Swaan. Membrane topology of human ASBT (SLC10A2) determined by dual label epitope insertion scanning mutagenesis. New evidence for seven transmembrane domains. Biochemistry 45:943–953 (2006).
A. Q. Sun, M. Ananthanarayanan, C. J. Soroka, S. Thevananther, B. L. Shneider, and F. J. Suchy. Sorting of rat liver and ileal sodium-dependent bile acid transporters in polarized epithelial cells. Am. J. Physiol. 275:G1045–G1055 (1998).
A. Q. Sun, R. Salkar, Sachchidanand, S. Xu, L. Zeng, M. M. Zhou, and F. J. Suchy. A 14-amino acid sequence with a beta-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J. Biol. Chem. 278:4000–4009 (2003).
A. Banerjee, A. Ray, C. Chang, and P. W. Swaan. Site-directed mutagenesis and use of bile acid-MTS conjugates to probe the role of cysteines in the human apical sodium-dependent bile acid transporter (SLC10A2). Biochemistry 44:8908–8917 (2005).
E. Y. Zhang, M. A. Phelps, A. Banerjee, C. M. Khantwal, C. Chang, F. Helsper, and P. W. Swaan. Topology scanning and putative three-dimensional structure of the extracellular binding domains of the apical sodium-dependent bile acid transporter (SLC10A2). Biochemistry 43:11380–11392 (2004).
W. Kramer, F. Girbig, H. Glombik, D. Corsiero, S. Stengelin, and C. Weyland. Identification of a ligand-binding site in the Na+/bile acid cotransporting protein from rabbit ileum. J. Biol. Chem. 276:36020–36027 (2001).
W. Kramer, K. Sauber, K. H. Baringhaus, M. Kurz, S. Stengelin, G. Lange, D. Corsiero, F. Girbig, W. Konig, and C. Weyland. Identification of the bile acid-binding site of the ileal lipid-binding protein by photoaffinity labeling, matrix-assisted laser desorption ionization-mass spectrometry, and NMR structure. J. Biol. Chem. 276:7291–7301 (2001).
N. Hussainzada, A. Banerjee, and P. W. Swaan. Transmembrane domain VII of the human apical sodium-dependent bile acid transporter ASBT (SLC10A2) lines the substrate translocation pathway. Mol. Pharmacol. 70:1565–1574 (2006).
P. A. Dawson, J. Haywood, A. L. Craddock, M. Wilson, M. Tietjen, K. Kluckman, N. Maeda, and J. S. Parks. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J. Biol. Chem. 278:33920–33927 (2003).
B. G. Bhat, S. R. Rapp, J. A. Beaudry, N. Napawan, D. N. Butteiger, K. A. Hall, C. L. Null, Y. Luo, and B. T. Keller. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J. Lipid Res. 44:1614–1621 (2003).
K. Kitayama, D. Nakai, K. Kono, A. G. van der Hoop, H. Kurata, E. C. de Wit, L. H. Cohen, T. Inaba, and T. Kohama. Novel non-systemic inhibitor of ileal apical Na+-dependent bile acid transporter reduces serum cholesterol levels in hamsters and monkeys. Eur. J. Pharmacol. 539:89–98 (2006).
P. Oelkers, L. C. Kirby, J. E. Heubi, and P. A. Dawson. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J. Clin. Invest. 99:1880–7 (1997).
B. L. Shneider. Intestinal bile acid transport: biology, physiology, and pathophysiology. J. Pediatr. Gastroenterol. Nutr. 32:407–417 (2001).
D. Jung, M. Fried, and G. A. Kullak-Ublick. Human apical sodium-dependent bile salt transporter gene (SLC10A2) is regulated by the peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 277:30559–30566 (2002).
F. Chen, L. Ma, N. Al-Ansari, and B. Shneider. The role of AP-1 in the transcriptional regulation of the rat apical sodium-dependent bile acid transporter. J. Biol. Chem. 276:38703–38714 (2001).
E. Neimark, F. Chen, X. Li, M. S. Magid, T. M. Alasio, T. Frankenberg, J. Sinha, P. A. Dawson, and B. L. Shneider. c-Fos is a critical mediator of inflammatory-mediated repression of the apical sodium-dependent bile acid transporter. Gastroenterology 131:554–567 (2006).
C. Thomas, J. F. Landrier, D. Gaillard, J. Grober, M. C. Monnot, A. Athias, and P. Besnard. Cholesterol-dependent down-regulation of mouse and human apical sodium-dependent bile acid transporter (ASBT) gene expression: molecular mechanism and physiological consequences. Gut 55(9):1321–1331 (2006).
W. A. Alrefai, F. Annaba, Z. Sarwar, A. Dwivedi, S. Saksena, A. Singla, P. K. Dudeja, and R. K. Gill. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: Role of sterol regulatory element binding protein 2. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G369–G376 (2007).
J. D. Horton, J. L. Goldstein, and M. S. Brown. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109:1125–1131 (2002).
M. B. Katanand A. C. Beynen. Characteristics of human hypo- and hyperresponders to dietary cholesterol. Am. J. Epidemiol. 125:387–399 (1987).
D. J. McNamara, R. Kolb, T. S. Parker, H. Batwin, P. Samuel, C. D. Brown, and E. H. Ahrens, Jr. Heterogeneity of cholesterol homeostasis in man. Response to changes in dietary fat quality and cholesterol quantity. J. Clin. Invest. 79:1729–1739 (1987).
M. Arrese, M. Trauner, R. J. Sacchiero, M. W. Crossman, and B. L. Shneider. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology 28:1081–1087 (1998).
A. Figge, F. Lammert, B. Paigen, A. Henkel, S. Matern, R. Korstanje, B. L. Shneider, F. Chen, E. Stoltenberg, K. Spatz, F. Hoda, D. E. Cohen, and R. M. Green. Hepatic overexpression of murine Abcb11 increases hepatobiliary lipid secretion and reduces hepatic steatosis. J. Biol. Chem. 279:2790–2799 (2004).
E. Neimark, F. Chen, X. Li, and B. L. Shneider. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology 40:149–156 (2004).
F. Chen, L. Ma, P. A. Dawson, C. J. Sinal, E. Sehayek, F. J. Gonzalez, J. Breslow, M. Ananthanarayanan, and B. L. Shneider. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 278:19909–19916 (2003).
F. Chen, L. Ma, R. B. Sartor, F. Li, H. Xiong, A. Q. Sun, and B. Shneider. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology 123:2005–2016 (2002).
U. Sundaram, S. Wisel, S. Stengelin, W. Kramer, and V. Rajendran. Mechanism of inhibition of Na+-bile acid cotransport during chronic ileal inflammation in rabbits. Am. J. Physiol. 275:G1259–G1265 (1998).
M. J. Nowicki, B. L. Shneider, J. M. Paul, and J. E. Heubi. Glucocorticoids upregulate taurocholate transport by ileal brush-border membrane. Am. J. Physiol. 273:G197–G203 (1997).
J. E. Heubi and T. D. Gunn. The role of glucocorticoids in the postnatal development of ileal active bile salt transport. Pediatr. Res. 19:1147–1151 (1985).
D. Jung, A. C. Fantin, U. Scheurer, M. Fried, and G. A. Kullak-Ublick. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut 53:78–84 (2004).
X. Chen, F. Chen, S. Liu, H. Glaeser, P. A. Dawson, A. F. Hofmann, R. B. Kim, B. L. Shneider, and K. S. Pang. Transactivation of rat apical sodium-dependent bile acid transporter and increased bile acid transport by 1alpha,25-dihydroxyvitamin D3 via the vitamin D receptor. Mol. Pharmacol. 69:1913–1923 (2006).
I. Monteiro, E. S. David, and R. P. Ferraris. Ontogenetic development of rat intestinal bile acid transport requires thyroxine but not corticosterone. Pediatr. Res. 55:611–621 (2004).
A. D. Mottino, T. Hoffman, P. A. Dawson, M. G. Luquita, J. A. Monti, E. J. Sanchez Pozzi, V. A. Catania, J. Cao, and M. Vore. Increased expression of ileal apical sodium-dependent bile acid transporter in postpartum rats. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G41–G50 (2002).
A. Reymann, W. Braun, C. Drobik, and C. Woermann. Stimulation of bile acid active transport related to increased mucosal cyclic AMP content in rat ileum in vitro. Biochim. Biophys. Acta 1011:158–164 (1989).
J. H. Schlattjan, S. Benger, A. Herrler, U. von Rango, and J. Greven. Regulation of taurocholate transport in freshly isolated proximal tubular cells of the rat kidney by protein kinases. Nephron. Physiol. 99:35–42 (2005).
G. Alpini, S. Glaser, L. Baiocchi, H. Francis, X. Xia, and G. Lesage. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology 41:1037–1045 (2005).
X. Xia, M. Roundtree, A. Merikhi, X. Lu, S. Shentu, and G. Lesage. Degradation of the apical sodium-dependent bile acid transporter by the ubiquitin-proteasome pathway in cholangiocytes. J. Biol. Chem. 279:44931–44937 (2004).
Z. Sarwar, R. K. Gill, A. Borthakur, K. Ramaswamy, P. K. Dudeja, G. Hecht, and W. A. Alrefai. Ileal Apical Bile Acid Transporter activity is inhibited by enteropathogenic E.Coli (EPEC) infection. Gastroenterology 130:A100 (2006).
W. A. Alrefai, Z. Sarwar, F. Annaba, S. Saksena, A. Dwivedi, P. K. Dudeja, and R. K. Gill. Evidence for the association of Apical Ileal Bile Acid Transporter (ASBT) with lipid rafts. Gastroenterology 130:A100 (2006).
H. C. Walters, A. L. Craddock, H. Fusegawa, M. C. Willingham, and P. A. Dawson. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. Am. J. Physiol. Gastrointest. Liver. Physiol. 279:G1188–G1200 (2000).
A. Amelsberg, C. Jochims, C. P. Richter, R. Nitsche, and U. R. Folsch. Evidence for an anion exchange mechanism for uptake of conjugated bile acid from the rat jejunum. Am. J. Physiol. 276:G737–G742 (1999).
M. C. Lin, W. Kramer, and F. A. Wilson. Identification of cytosolic and microsomal bile acid-binding proteins in rat ileal enterocytes. J. Biol. Chem. 265:14986–95 (1990).
G. P. Tochtrop, K. Richter, C. Tang, J. J. Toner, D. F. Covey, and D. P. Cistola. Energetics by NMR: site-specific binding in a positively cooperative system. Proc. Natl. Acad. Sci. U. S. A. 99:1847–1852 (2002).
O. Toke, J. D. Monsey, G. T. DeKoster, G. P. Tochtrop, C. Tang, and D. P. Cistola. Determinants of cooperativity and site selectivity in human ileal bile acid binding protein. Biochemistry 45:727–737 (2006).
I. Zaghini, J. F. Landrier, J. Grober, S. Krief, S. A. Jones, M. C. Monnot, I. Lefrere, M. A. Watson, J. L. Collins, H. Fujii, and P. Besnard. Sterol regulatory element-binding protein-1c is responsible for cholesterol regulation of ileal bile acid-binding protein gene in vivo. Possible involvement of liver-X-receptor. J. Biol. Chem. 277:1324–1331 (2002).
T. Kanda, L. Foucand, Y. Nakamura, I. Niot, P. Besnard, M. Fujita, Y. Sakai, K. Hatakeyama, T. Ono, and H. Fujii. Regulation of expression of human intestinal bile acid-binding protein in Caco-2 cells. Biochem. J. 330(Pt 1):261–265 (1998).
J. Grober, I. Zaghini, H. Fujii, S. A. Jones, S. A. Kliewer, T. M. Willson, T. Ono, and P. Besnard. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J. Biol. Chem. 274:29749–29754 (1999).
T. Kok, C. V. Hulzebos, H. Wolters, R. Havinga, L. B. Agellon, F. Stellaard, B. Shan, M. Schwarz, and F. Kuipers. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J. Biol. Chem. 278:41930–41937 (2003).
M. Nakahara, N. Furuya, K. Takagaki, T. Sugaya, K. Hirota, A. Fukamizu, T. Kanda, H. Fujii, and R. Sato. Ileal bile acid-binding protein, functionally associated with the farnesoid X receptor or the ileal bile acid transporter, regulates bile acid activity in the small intestine. J. Biol. Chem. 280:42283–42289 (2005).
J. F. Landrier, J. Grober, J. Demydchuk, and P. Besnard. FXRE can function as an LXRE in the promoter of human ileal bile acid-binding protein (I-BABP) gene. FEBS Lett. 553:299–303 (2003).
J. F. Landrier, C. Thomas, J. Grober, I. Zaghini, V. Petit, H. Poirier, I. Niot, and P. Besnard. The gene encoding the human ileal bile acid-binding protein (I-BABP) is regulated by peroxisome proliferator-activated receptors. Biochim. Biophys. Acta. 1735:41–49 (2005).
M. D. Halpern, H. Holubec, T. A. Saunders, K. Dvorak, J. A. Clark, S. M. Doelle, N. Ballatori, and B. Dvorak. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130:359–372 (2006).
S. Sakamoto, H. Suzuki, H. Kusuhara, and Y. Sugiyama. Efflux mechanism of taurocholate across the rat intestinal basolateral membrane. Mol. Pharm. 3:275–281 (2006).
M. C. Lin, S. L. Weinberg, W. Kramer, G. Burckhardt, and F. A. Wilson. Identification and comparison of bile acid-binding polypeptides in ileal basolateral membrane. J. Membr. Biol. 106:1–11 (1988).
M. C. Lin, E. Mullady, and F. A. Wilson. Timed photoaffinity labeling and characterization of bile acid binding and transport proteins in rat ileum. Am. J. Physiol. 265:G56–G62 (1993).
W. Wang, D. J. Seward, L. Li, J. L. Boyer, and N. Ballatori. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc. Natl. Acad. Sci. U. S. A. 98:9431–9436 (2001).
D. J. Seward, A. S. Koh, J. L. Boyer, and N. Ballatori. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J. Biol. Chem. 278:27473–27482 (2003).
N. Ballatori, W. V. Christian, J. Y. Lee, P. A. Dawson, C. J. Soroka, J. L. Boyer, M. S. Madejczyk, and N. Li. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42:1270–1279 (2005).
J. F. Landrier, J. J. Eloranta, S. R. Vavricka, and G. A. Kullak-Ublick. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am. J. Physiol. Gastrointest. Liver. Physiol. 290:G476–G485 (2006).
M. Okuwaki, T. Takada, Y. Iwayanagi, S. Koh, Y. Kariya, H. Fujii, and H. Suzuki. LXR Alpha Transactivates Mouse Organic Solute Transporter Alpha and Beta via IR-1 Elements Shared with FXR. Pharm. Res. 24:390–398 (2007).
X. Xia, H. Francis, S. Glaser, G. Alpini, and G. LeSage. Bile acid interactions with cholangiocytes. World J. Gastroenterol. 12:3553–3563 (2006).
J. H. Schlattjan, C. Winter, and J. Greven. Regulation of renal tubular bile acid transport in the early phase of an obstructive cholestasis in the rat. Nephron. Physiol. 95:49–56 (2003).
Acknowledgments
The authors are thankful to Drs Pradeep K. Dudeja, K. Ramaswamy for their invaluable suggestions and critical reading of the manuscript. Thanks are also due to Dr. Fadi Annaba for his help in the preparation of this review. Studies in the authors laboratory are supported by NIDDK grant DK71596 (WAA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alrefai, W.A., Gill, R.K. Bile Acid Transporters: Structure, Function, Regulation and Pathophysiological Implications. Pharm Res 24, 1803–1823 (2007). https://doi.org/10.1007/s11095-007-9289-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9289-1