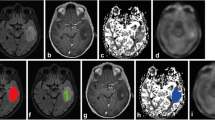
Abstract
Discriminating tumor infiltrative and vasogenic brain edema in malignant gliomas is important although challenging in clinical settings. This study challenged this issue by performing voxel-wise analysis of 18F-fluorodeoxy glucose (FDG) and 11C-methionine positron emission tomography (PET) in peritumoral brain edemas. The authors studied ten malignant glioma and nine meningioma patients with peritumoral brain edema. A voxel-wise analysis of FDG and 11C-methionine PET was performed in order to quantify the correlation between uptake of these tracers in normal brain tissue and peritumoral brain edema. Decoupling score of the uptake of two tracers was calculated as the z-score from the estimated correlation between uptake of the two tracers in normal brain tissue. The decoupling score was also converted into images for visual inspection. Average decoupling score in the peritumoral brain edema was calculated and compared between those obtained from malignant gliomas and meningiomas. FDG and 11C-methionine uptake showed a reproducible linear correlation in normal brain tissue. This correlation was preserved in peritumoral edema of meningioma, but not in that of malignant gliomas. In malignant gliomas, higher 11C-methionine uptake compared to that estimated by the FDG uptake in normal brain tissue was observed, thus suggesting that decoupling was caused by tumor infiltration. Visual inspection of the decoupling score enabled discrimination of tumor infiltrative and vasogenic edema. The average decoupling scores of the peritumoral brain edema in malignant gliomas were significantly higher than those in meningiomas (2.9 vs. 0.7, P = 0.0003). As a conclusion, FDG/11C-methionine uptake decoupling score can be used for the discrimination of tumor infiltrative and vasogenic brain edema. The proposed method also suggests the possibility of accurately detecting tumor infiltration into brain tissues in gliomas, providing significant information for treatment planning and follow-up.









Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DTI:
-
Diffusion tenor imaging
- FA:
-
Fractional anisotropy
- FDG:
-
18F-fluorodeoxy glucose
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- VOI:
-
Voxels of interest
References
Kinoshita M, Goto T, Okita Y, Kagawa N, Kishima H, Hashimoto N, Yoshimine T (2010) Diffusion tensor-based tumor infiltration index cannot discriminate vasogenic edema from tumor-infiltrated edema. J Neurooncol 96:409–415
Kinoshita M, Hashimoto N, Goto T, Yanagisawa T, Okita Y, Kagawa N, Kishima H, Tanaka H, Fujita N, Shimosegawa E, Hatazawa J, Yoshimine T (2009) Use of fractional anisotropy for determination of the cut-off value in 11C-methionine positron emission tomography for glioma. Neuroimage 45:312–318
Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI (2004) Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 232:221–228
Stadlbauer A, Ganslandt O, Buslei R, Hammen T, Gruber S, Moser E, Buchfelder M, Salomonowitz E, Nimsky C (2006) Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 240:803–810
Stadlbauer A, Nimsky C, Buslei R, Salomonowitz E, Hammen T, Buchfelder M, Moser E, Ernst-Stecken A, Ganslandt O (2007) Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. Neuroimage 34:949–956
Lau EW, Drummond KJ, Ware RE, Drummond E, Hogg A, Ryan G, Grigg A, Callahan J, Hicks RJ (2010) Comparative PET study using F-18 FET and F-18 FDG for the evaluation of patients with suspected brain tumour. J Clin Neurosci 17:43–49
Herholz K, Holzer T, Bauer B, Schroder R, Voges J, Ernestus RI, Mendoza G, Weber-Luxenburger G, Lottgen J, Thiel A, Wienhard K, Heiss WD (1998) 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 50:1316–1322
Kracht LW, Friese M, Herholz K, Schroeder R, Bauer B, Jacobs A, Heiss WD (2003) Methyl-[11C]- l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 30:868–873
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, Klein JC, Herholz K, Heiss WD (2004) Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10:7163–7170
Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishiwata K, Senda M, Ishii K, Hirakawa K, Ohno K (2005) Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 103:498–507
Okita Y, Kinoshita M, Goto T, Kagawa N, Kishima H, Shimosegawa E, Hatazawa J, Hashimoto N, Yoshimine T (2010) (11)C-methionine uptake correlates with tumor cell density rather than with microvessel density in glioma: a stereotactic image-histology comparison. Neuroimage 49:2977–2982
Berger G, Maziere M, Knipper R, Prenant C, Comar D (1979) Automated synthesis of 11C-labelled radiopharmaceuticals: imipramine, chlorpromazine, nicotine and methionine. Int J Appl Radiat Isot 30:393–399
Veninga T, Huisman H, van der Maazen RW, Huizenga H (2004) Clinical validation of the normalized mutual information method for registration of CT and MR images in radiotherapy of brain tumors. J Appl Clin Med Phys 5:66–79
Yang I, Aghi MK (2009) New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol 6:648–657
Lin X, Tang Y, Sun B, Hou Z, Meng H, Li Z, Liu Q, Liu S (2010) Cerebral glucose metabolism: Influence on perihematomal edema formation after intracerebral hemorrhage in cat models. Acta Radiol 51:549–554
Pauleit D, Stoffels G, Bachofner A, Floeth FW, Sabel M, Herzog H, Tellmann L, Jansen P, Reifenberger G, Hamacher K, Coenen HH, Langen KJ (2009) Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nucl Med Biol 36:779–787
Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H, Yoshimura S, Maruyama T, Muragaki Y, Iwama T (2008) Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. Am J Neuroradiol 29:1176–1182
Yamamoto Y, Nishiyama Y, Kimura N, Kameyama R, Kawai N, Hatakeyama T, Kaji M, Ohkawa M (2008) 11C-acetate PET in the evaluation of brain glioma: comparison with 11C-methionine and 18F-FDG-PET. Mol Imaging Biol 10:281–287
Iuchi T, Hatano K, Narita Y, Kodama T, Yamaki T, Osato K (2006) Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys 64:1317–1324
Acknowledgments
This investigation was supported by the Osaka Cancer Research Foundation, the Konica Minolta Imaging Science Foundation, the Osaka Cancer Researcher Training Fund, the Takeda Science Foundation, the Sagawa Foundation for Promotion of Cancer Research and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kinoshita, M., Goto, T., Arita, H. et al. Imaging 18F-fluorodeoxy glucose/11C-methionine uptake decoupling for identification of tumor cell infiltration in peritumoral brain edema. J Neurooncol 106, 417–425 (2012). https://doi.org/10.1007/s11060-011-0688-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0688-0