Abstract
Stroke can cause permanent neurological damage, complications, and even death. However, there is no treatment exists to restore its lost function. Human embryonic stems transplantation therapy was a novel and potential therapeutic approach for stroke. However, as we have seen, the ethical controversy pertains to embryonic stem cell research. Human induced pluripotent stem cells (iPSCs) are the latest generation of stem cells that may be a solution to the controversy of using embryonic cells. In our study, we generated iPSCs from adult human fibroblasts by introduction of four defined transcription factors (Oct4, Sox2, Nanog, and Lin-28). And then, we investigated the efficacy of iPSCs transplantation therapy for stroke on the animal models of middle cerebral artery occlusion. Surprisingly, we found that transplanted iPSCs migrated to injured brain areas, and differentiated into neuron-like cells successfully. After 4–16 days iPSCs grafting, sensorimotor function of rats has been improved significantly. In one word, we may prove that iPSCs therapy in stroke to be an effective form of treatment.
Similar content being viewed by others
Introduction
Stroke is currently the leading cause of adult disability in the United States with over 700,000 Americans affected per year [1]. Furthermore, the number of chronic stroke presentations is projected to increase over the next 30–40 years. However, there is no more effective therapeutic method for treatment of stroke. Recently, a novel approach to the treatment of stroke is to promote functional brain recovery through direct cell transplantation into the central nervous system (CNS). In addition, many groups have been shown that embryonic stem cells have successfully demonstrated functional recovery in animal models [2–7].
Embryonic stem cells proliferate rapidly while maintaining pluripotency, namely, the ability to differentiate into various types of cells [8–10]. Clinical application of human embryonic stem cells faces difficulties regarding technical, ethical, or immunological considerations. One way to circumvent these issues is to generate pluripotent cells directly from somatic cells nuclear transfer (SCNT) to oocytes and fusion with ES cells [11, 12]. Another study showed that fusion-mediated reprogramming is facilitated by overexpressing the transcription factor Nanog in embryonic stem (ES) cells. However, these methods still require embryos or oocytes to generate pluripotent cells thus are not free from ethical issues. In addition, fusion-mediated methods require elimination of ES-cell-derived chromosomes.
Another way to circumvent these issues is to induce pluripotent status in somatic cells by direct reprogramming [12]. An approach toward the same end was recently described, in which murine fibroblasts were reprogrammed by ectopically expressing factors known to be highly expressed in murine ES cells [13, 14]. Two sets of four-factors, OCT4, SOX2, C-MYC, and KLF4 reported by Yamanaka’s laboratory and OCT4, SOX2, NANOG, and LIN28 reported by Thomson’s laboratory, have been shown to reprogram human somatic cells to pluripotency with similar efficiency (10–20 iPSCs colonies from 0.1 million initial fibroblasts) [15, 16].
Successful reprogramming of differentiated human somatic cells into a pluripotent state would allow creation of patient- and disease-specific stem cells. The generation of iPSCs represents a major advance in the field of regenerative medicine and provides a powerful tool for the study of cell-fate transitions. The generation of patient-specific pluripotent stem cells has the potential to accelerate the implementation of stem cells for clinical treatment of degenerative diseases. In our study, we reprogrammed human fibroblast cells into iPSCs, and then, we also demonstrate their long-term expansion and functional engraft ability in an experimental model of stroke.
Materials and methods
Derivation and characterization of iPSCs
Human fibroblast was obtained from a male patient (62 years old) of Shanghai Pudong New Area Gongli Hospital. All of the human samples were obtained after approval from the Ethical Review Board of the Pudong New Area Gongli Hospital and after obtaining written informed consent from subjects.
For lentivirus production and generation of iPSCs, the retroviral-expression vector (with EGFP) of encoding human OCT4, SOX2, NANOG, and LIN-28 were co-transfected with packing plasmids GAG, POL, and VSVG into 293T cells with using the FuGENE 6 kit (Roche). Forty-eight hours after transfection, the supernatant of transfectant was collected and filtered through a 0.45 mm pore-size cellulose acetate filter (Whatman). Human fibroblasts were seeded at 8 × 105 cells per 100 mm dish 1 day before transduction. The medium was replaced with virus-containing supernatant supplemented with 4 mg/ml polybrene (Nacalai Tesque), and incubated for 24 h. Twenty-four hours later, infected fibroblast cells were replanted onto irradiated mouse embryonic fibroblasts (MEFs) cells with the standard human ES cell culture medium as previously reported [17]. 7 days later, small colonies emerged in the culture dish. Single colonies were picked up 2 days later and cultured until they grew to large colonies.
Establishment of animal model and cell transplantation
Female Sprague–Dawley rats (230–270 g) were housed for 7 days prior to occlusion, with food available ad libitum. Thirty rats were prepared for surgery and subjected to 70 min of middle cerebral artery occlusion (MCAO) [18–20] by using a 3.0-prolene filament coated with silicone. Halothane (in 70% NO2/30% O2) anesthesia was used for insertion and removal of the filament, with temperatures held at 37 ± 1°C by rectal probe and heating pad. Once the filament was inserted (20 mm), animals were allowed to recover from anesthesia. At 60 min of the occlusion, rats were evaluated for behavioral dysfunction (forelimb flexion and contralateral circling behavior). At the end of the occlusion period, the rats were re-anesthetized and clear the base of the MCA. After the wound was closed, rats placed in heated chamber for 2 h. All rats were scored for neurological dysfunction and weighed daily. Rehydration therapy was administered as necessary in cases with depressed body weight. Once preoperative body weight was reached, animals were tested on the tape removal [21] and rotameter tasks to provide baseline scores for the test battery and to allow for the establishment of balanced experimental groups.
MCAO rats were divided to 3 groups (treated with PBS, treated with primary fibroblast cells (MCS), treated with iPSCs, 10 rats/group). After preparation, rats were placed into stereotaxic frames, the scalp incised, skull exposed and bregma located. Grafts (2 μl of cell suspension or vehicle) were deposited using a flat tipped 10 μl Hamilton syringe at 4 sites, ipsilateral and contralateral to the occluded hemisphere at the following coordinates relative to bregma. Cells were injected at a rate of 1 μl/min and the syringe kept in place for another minute to reduce reflux. The injection position was 3 mm ventral to the skull, 0.8 mm posterior, and 2 mm lateral to bregma. Once all cells were injected (each = 800,000), the wounds were sutured shut and animals were given saline rehydration, and anti-inflammatory treatment. Animals received post-operative immunosuppression treatment with medrone daily for 20 days over the course of the study.
Quantitative reverse transcription polymerase chain reaction (PCR) analyses
Total RNA was extracted from cells with using Trizol reagent (Invitrogen) and DNase I (Promega). The cDNA synthesis was performed with using Superscript II reverse transcriptase (Invitrogen). 1 μg of DNase I-treated total RNA as template in a 20 μl reaction volume. For quantitative gene expression analysis, 1 μl cDNA was subjected to PCR amplification by using SYBR Green I (Invitrogen) on the ABI Prism 7700 system (Applied Biosystems, U.S.A.) for real-time detection of PCR products. Product amplification was performed with initial polymerase activation (5 min at 95°C) followed by 40 two-step PCR cycles comprising denaturing (15 s at 95°C), annealing and extension (1 min at 60°C). The housekeeping gene GAPDH served as the internal reference and was amplified parallel to the gene of interest for every template. Mean mRNA ratios, representing gene expression relative to the GAPDH reference gene, were calculated as the means of five individual mRNA ratios. Primers of genes are shown in Table 1.
Immunofluorescence staining
Cells on the glass cover slips were fixed in 4% paraformaldehyde in PBS supplemented with 0.1% Triton-X-100 for 15 min at room temperature (RT), followed by permeabilization with PBS with 0.1% Triton-X-100 for 5 min at RT. Cells were blocked for 30 min in PBS with 3% BSA. All primary antibodies were diluted in the same blocking buffer and incubated with samples overnight at 4°C (NANOG, OCT4, SOX2, GFAP, Nestin and Vimentin, first antibody diluted 1:200). Cells treated with fluorescently coupled secondary antibody (anti-rabbit Cy3, Jackson ImmunoResearch, 1:200) were incubated for 1 h at RT. All images were captured using confocol microscopy with magnification 100×.
Histological evaluation
Animals were overdosed with pentobarbital and transcardially perfused at a pressure of 160 mm Hg with 25 ml of PBS containing sodium heparin (25 U/ml), followed immediately by 250 ml of 4% paraformaldehyde in PBS. Brains were dissected out and stored in the same fixative for 4 h at room temperature. They were then transferred to 30% sucrose in PBS and stored at 4°C.
Indirect fluorescence immunohistochemical methods were used to identify the implanted iPSCs. Differentiation of transplanted cells was determined in serial sections by co-labeling with rabbit polyclonal antibodies against the neural phenotypic markers, GFAP for astroglia. Proliferative capacity of the implanted cells was assessed by colabeling with a rabbit polyclonal antibody against the neuroepithelial stem cell marker Nestin and the intermediate filament protein, Vimentin. Primary antibodies were incubated overnight before the fluorescent Rhodamine-conjugated goat anti-rabbit secondary antibodies were applied for 2 h. Sections were initially examined on a Leica DMRB microscope fitted with epifluorescence optics followed by more detailed examinations and imaging on a confocal microscope (Leica TCS NT).
Percentage hemisphere lesion volume (%HLV) and Neurologic deficit
At 2 and 16 days after transplant, the rats were killed under deep anesthesia with an overdose of chloral hydrate. To quantify ischemic damage, brains were dissected and cut into 5 coronal slices of 2-mm thickness, incubated in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C for 15 min and immersion-fixed in a 4% paraformaldehyde. TTC-stained sections were photographed and the digital images were analyzed using image analysis software (AutoCAD). To compensate for the effect of brain edema, %HLV was calculated by the following equations, based on that used by Li et al. [22]. %HLV = {[total infarct volume − (right hemisphere volume − left hemisphere volume)]/left hemisphere volume} × 100%.
A neurologic test was carried out by an examiner blinded to the experimental groups at 0, 4, 8, 12, and 16 days after transplant, all of 6 rats from each group were assessed on a modified scoring system that developed from Longa et al. [20], as follows: 0, no deficits; 1, difficulty in fully extending the contralateral forelimb; 2, unable to extend the contralateral forelimb; 3, mild circling to the contralateral side; 4, severe circling and 5, falling to the contralateral side.
Statistical analysis
Outcome measurement for each experiment was reported as Mean ± SEM. All data were analyzed using SPSS 11 for Mac OS X (SPSS Inc.). Significance of inter-group differences was performed by applying Student’s t-test where appropriate.
Results
Generation and characterization of iPSCs in vitro
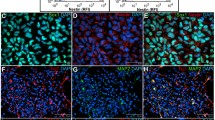
The adult human fibroblasts cells were transfected with Lentivirus-iPSCs gene, which has four defined transcription factors: OCT4, SOX2, NANOG, and LIN28. After culturing for 7 days, the primary cell clones have been formed (Fig. 1A, B) and have digested and plated above the MEF cells. 4 days later, several ES-like clones appeared (Fig. 1C) and detected by positive immunostaining of Oct4 (Fig. 1D), Nanog (Fig. 1E), and Sox2 (Fig. 1F). Figure 1D–F showed that these reprogrammed cells started to express the markers of stem cells (Oct4, Nanog and Sox2), which suggested that human fibroblasts could be transformed into the iPSCs successfully.
Characterization of iPSCs. The adult human fibroblasts cells were transected with four defined transcription factors: OCT4, SOX2, NANOG and LIN28. After 7 days, the primary cell clones have been formed (A, B) and have digested and plated above the MEF feeding cells. 4 days later, several ES-like clones appeared (C). D–F showed that these reprogrammed cells started to express the markers of stem cells (Oct4, Nanog and Sox2) (Magnification × 100)
Furthermore, we analyzed the presence of the transcription factor Nanog, Otc-4, Sox2, c-Myc, and KLF-4 in candidate cell clones by real-time PCR analysis (Fig. 2A). As shown in Fig. 2A, the third cell clone (iPSC-3) was identified as positive with ES markers (Nanog, Otc-4, Sox-2, c-Myc, and KLF-4). It is also found this clone expressed alkaline phosphatase (Fig. 2B).
Therefore, we further expanded this clone and evaluated with a range of assays to identify key biological of this cell line in vitro. Furthermore, we found the expression of endogenous Oct4, Nanog, Rex-1, and Sox2 were robustly induced in iPSC-3, which were similar to ES cells (Fig. 3A). These observations suggested that endogenous pluripotency genes (e.g., Oct4, Nanog REX, and Sox2) could be fully induced by reprogramming of the fibroblast cell.
Differentiation potent of iPSCs. Expression of stem cell and differentiation markers was detected in 4 clones of iPSCs (clones 1, 2, 3, 4) by qRT-PCR. Clone iPSC-3 cells shows to differentiate into all three germ layers in embryoid bodies, as evidenced by the expression of AFP (endoderm), Amylase (endoderm), Enolase (mesoderm), Osteonectin (mesoderm), NeuroD (ectoderm) and GFAP (ectoderm) (A). Hematoxylin-eosin staining of teratomas derived from iPSC-3 is composed of various types of tissues: B gut-like epithelium (endoderm), C bone (mesoderm) and D neuroepithelium (ectoderm). (Magnification ×100)
As we known, iPSCs are able to differentiate into three germ layers in vitro and in vivo. In order to determine the differentiation capacity of iPSC-3 cells in vitro, we induced the iPSC-3 cells to differentiate for 7 days and analyzed the presence of differentiation markers. RT–PCR analysis confirmed that the iPS3 cells could differentiate into all three germ layers in embryoid bodies, as evidenced by the expression of AFP (endoderm), Amylase (endoderm), Osteonectin (mesoderm) and GFAP (ectoderm) (Fig. 3A).
To test pluripotency of iPSC-3 in vivo, iPSC-3 cells were injected intramuscularly into non-obese diabetic/severe combined immune deficient (NOD/SCID) mice. Four weeks after injection, we observed tissues contain three germ layers, including gut-like epithelial tissues (endoderm), bone (mesoderm) and neural tissues (ectoderm) (Fig. 3B–D). These data demonstrate that iPSCs can differentiate into three germ layers in vitro and in vivo.
Transplanted iPSCs survived, engrafted into the stroke-damaged host tissue and differentiated into neural cells
To investigate the survival and functional engraftment in an injury environment, iPSCs (8 × 105) were transplanted into the ischemic boundary zone in the rat striatum 1 week after the MCAO was performed (Fig. 4A). Four weeks later, we found that EGFP positive cells in the ischemic rat brain, which demonstrated that the transplanted iPSCs can survive in the ischemic rat brain. For the iPSCs migration, Fig. 4B, C showed that morphology of graft iPSCs changed significantly after 3 weeks transplantation. It means that iPSCs might migrate to ischemic rat brain.
iPSCs could differentiate into neural cells in vivo. A diagram showed the iPSCs were grafted into the cortex surrounding the infarct area following cortical ischemia (A). 16 days after transplantation, coronal cryostat sections were processed for immunohistochemistry. GFP-positive cells were found at the site of grafting, and along the two migratory pathways (B, C)
To investigate whether iPSCs could different into neural cells, we detected expression of neural cell markers in graft area. We found some GFAP antibody labeled cells in the cortex area (Fig. 5A), Nestin positive cells in striatum area (Fig. 5B), and Vimentin positive cells in ependymocytes (Fig. 5C). These results suggest that injected iPSCs could migrate in the rat brain and differentiate into neural cells. However, it is unclear that mechanism of iPSCs migration and differentiation. It is that microenvironments may be potential factors for iPSCs differentiation.
Transplanted cells improve sensorimotor and recovery function
In order to assess therapeutic benefit of iPSCs, the cells were tested in a temporary occlusion model of stroke. It is found that grafting of cells did not produce any seizure or convulsive activity. Moreover, percentage hemisphere lesion volume (%HLV) was reduced significantly after animal’s treatment of iPSCs. It is suggested that iPSCs could repair the injured areas of stroke and to enhance functional recovery (Fig. 6A).
Functional recovery in animal models. In order to assess therapeutic benefit of iPSCs, the cells were tested in a temporary occlusion model of stroke (MCAO). The treated animals (iPSCs) have a significantly reduction in percentage of hemisphere lesion volume when compared to vehicle treated animals (MCS Cell group) or control group (PBS group) (P < 0.01). A A neurologic test was carried out by an examiner blinded to the experimental groups. Behavioral tests commenced 4 days and ended 16 days post-grafting. The treated animals (iPSCs) have a significantly reduced score when compared to vehicle treated animals (MCS Cell group) (P < 0.01) (B)
Whether transplanted iPSCs could enhance the recovery of sensorimotor function, we used the cylinder test to measure the sensorimotor asymmetry in forelimb commenced 4 days and ended 16 days post-grafting. It is showed that the group of iPSCs grafting have a significantly reduction in scores when compared to vehicle treated animals with primary fibroblast cell group or control group (PBS group) (P < 0.01). It reminded us that iPSCs could help sensorimotor function recovery (Fig. 6B).
Discussion
In this study, we demonstrated the generation of induced pluripotent stem cells from adult human fibroblasts by lentiviral introduction of the four defined transcription factors. The iPSCs is colonial, conditionally immortal, and has been derived and may be a viable candidate for clinical trials. In addition, the iPSCs have been shown to be negative in an in vitro tumorigenesis assay and have not formed tumors after grafting into approximately 30 rats. This study demonstrated excellent survival of iPSCs in animals at both 2- and 16-day time points. Cells were seen to migrate from the injection tract over time. At 2 days post-implantation, grafted cells were predominantly located within the injection tract. By 16 days, cells survived and migrated in the corpus callosum and ventriculus lateralis of ischemic rat.
The human cell line evaluated in this study produced equivalent recovery in sensorimotor deficits as was seen in previous studies using the murine line. These data demonstrated that human stem cell therapy can also alleviate long-term sensorimotor deficits after stroke. The delay between stroke onset and grafting allowed time for the resolution of acute stroke pathology before the testing of sensorimotor deficits. The delay between occlusion and grafting has been partly utilized to extrapolate data to the clinical situation. Patients will need to recover from the acute pathology of a stroke and begin behavioral evaluations before recommendation of cell therapy. Probable long-term recovery from behavioral deficits and possible benefits of cell therapy could be ascertained in the clinic by 1-month postictus [23]. Preparation for grafting with imaging of the infarction, planning graft trajectories and scheduling for surgery will probably require several weeks before treatment with cell therapy. Few studies have examined the effects of grafting cells into “mature” strokes [21, 24, 25]. Acute treatments with i.v. infusion of bone marrow cells or umbilical cord cells are usually performed within 24–48 h of infarct with behavioral effects evident within a few days [23, 26, 27]. The acute effect of infusing cells, usually coupled with reduced infarct volumes, suggests that recovery with these strategies is due to neuroprotection, not restoration of function.
The bilateral asymmetry test results showed resolution of deficits with the grafted cell line. The combination of cortical and striatal effects implies that the cell line works at both cortical and subcortical levels to improve recovery. The simplest mechanisms that can be hypothesized for repair in cell transplantation approaches to treating neurodegenerative diseases are that either “grafted cells will replace lost tissue, like for like” or “grafted cells will induce favorable changes in host brain tissue”. In both scenarios, survival of grafted cells is prerequisite and in the former case differentiation into the right phenotype would be important in predicting positive outcome in behavioral studies. Cell transplants have shown efficacy in recovery from behavioral deficits in several models of neurodegeneration [4, 5, 7]. While transplants of dopaminergic cells in Parkinson’s disease have a theoretically simple mechanism of action in replenishing the levels of neurotransmitter to ease symptoms of motor dysfunction, mechanisms of recovery in the stroke model are not so easily explained. Other groups have also seen positive behavioral effects following cell grafting that were not dependent upon the formation of new neurons that were integrated into the neurons in the neuropil [5].
Previous studies with murine cell lines used similar doses of cells (approximately 2–6 × 105) in up to 12 μl of inoculate, setting a precedent of recovery of function without tissue replacement. Anticipated dosing in the clinic (approximately 25 to 50 million cells) would result in a volume of 0.4–0.8 ml of injectate, again, miniscule, when compared to the volume of a human brain (1,400 cm3). The present study replicated many of the parameters of the murine line studies with a similar cell concentration and multiple deposits. Multiple injection sites allow for the injection of the necessary volume of cells. If some substances are released, spreading the injections may aid in distribution across the brain [28]. Further study will investigate alteration of cell concentrations to determine the cell load necessary for efficacy. As yet, no reliable molecular markers of recovery potential for stroke cell therapy are evident, stressing the importance of in vivo behavioral testing for demonstrating clinical potential.
Conclusion
Here, we demonstrate the generation of induced pluripotent stem cells from adult human fibroblasts with defined transcription factors. The iPSCs is colonial, conditionally immortal, and has been derived and may be a viable candidate for clinical trials. This cell line has good growth characteristics and stable genetics. This study has shown that delayed grafting of this human stem cell line can promote functional recovery in adult rats after stroke. Improvements were seen in chronic sensorimotor deficits that involve both striatal and cortical input, indicating a promising method of clinical treatment.
Abbreviations
- CNS:
-
Central nervous system
- ES:
-
Embryonic stem
- %HLV:
-
Percentage hemisphere lesion volume
- HESC:
-
Human embryonic stem cells
- iPSCs:
-
Induced pluripotent stem cells
- MCAO:
-
Middle cerebral artery occlusion
- SCNT:
-
Somatic cells nuclear transfer
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
References
Adams H, Adams R, Del Zoppo G, Goldstein LB (2005) Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke 36:916–923
Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY (1998) Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol 16:1033–1039
Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN (2001) Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol 19:475–479
Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK (2004) Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA 101:11839–11844
Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY (2002) Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol 20:1103–1110
Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418:50–56
Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N (2005) Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 115:102–109
Yamanaka S (2008) Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif 41:51–56
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 174:11–20
Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S (2008) Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321:699–702
Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM (2007) Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450:497–502
Yamanaka S (2007) Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1:39–49
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K (2008) Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA 105:2883–2888
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Di Santo J, Weber A, Vallier L (2010) Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51:1754–1765
Allahtavakoli M, Moloudi R, Arababadi MK, Shamsizadeh A, Javanmardi K (2009) Delayed post ischemic treatment with Rosiglitazone attenuates infarct volume, neurological deficits and neutrophilia after embolic stroke in rat. Brain Res 1271:121–127
Li JS, Zhang W, Kang ZM, Ding SJ, Liu WW, Zhang JH, Guan YT, Sun XJ (2009) Hyperbaric oxygen preconditioning reduces ischemia-reperfusion injury by inhibition of apoptosis via mitochondrial pathway in rat brain. Neuroscience 159:1309–1315
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Veizovic T, Beech JS, Stroemer RP, Watson WP, Hodges H (2001) Resolution of stroke deficits following contralateral grafts of conditionally immortal neuroepithelial stem cells. Stroke 32:1012–1019
Li J, Henman MC, Tatlisumak T, Shaw GG, Doyle KM (2005) The pre-ischaemic neuroprotective effects of N1-dansyl-spermine in a transient focal cerebral ischaemia model in mice. Brain Res 1055:180–185
Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ (2003) Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 34:2181–2186
Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR (1998) Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol 149:310–321
Saporta S, Borlongan CV, Sanberg PR (1999) Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative dose-response analysis of cell survival and behavioral recovery. Neuroscience 91:519–525
Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, Kim SU, Roh JK (2004) Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res 1016:145–153
Willing AE, Vendrame M, Mallery J, Cassady CJ, Davis CD, Sanchez-Ramos J, Sanberg PR (2003) Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant 12:449–454
Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H (2000) Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. J Neurosci Methods 104:99–109
Acknowledgments
The authors would like to thank members of Professor Wu Xinzhong’s laboratory for their helpful discussion. This work was partially supported by Natural Science Foundation of China, grant number, 30570632. Pudong New Area, the leading health systems, medical personnel training programs, grant number, PWRq2007-03.
Conflict of interest
All authors have no competing interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mei Jiang and Lei Lv have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, M., Lv, L., Ji, H. et al. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem 354, 67–75 (2011). https://doi.org/10.1007/s11010-011-0806-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0806-5