Abstract
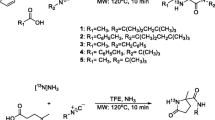
For experimental studies by animal PET [11C]-labeled 15-(4-methylphenyl)pentadecanoic acid (MePPA) is an attractive alternative to the radioiodinated 15-(4-iodophenyl)pentadecanoic acid (IPPA) which has widely been used for imaging of fatty acid metabolism. The important physiological aspect is that the iodine atom and the methyl substituent have similar steric and lipophilic properties. For preparation of [11C]MePPA, Stille cross-coupling reaction was applied since the same tin precursor as for the radiosynthesis of IPPA and readily available [11C]CH3I can be used. Unsaturated tris(dibenzylideneacetone)dipalladium(0)/tri(o-tolyl)phosphine [Pd2(dba)3/P(o-tolyl)3] was taken as the catalytic system. The reaction conditions were optimized with respect to temperature, time, solvent and amount of precursor. The best radiochemical yields of 73 ± 2.8% (decay corr.) were obtained using 0.525 mg tin precursor in DMF at 80 °C already after a reaction time of 10 min. The labeled methyl ester was hydrolyzed by 1 M NaOH/EtOH at 80 °C within 3 min to give [11C]IPPA in a RCY of 62 ± 3.0%. The radiochemical purity of the product assured by HPLC was >99% and the overall preparation time including HPLC purification and formulation was 40 min.



Similar content being viewed by others
References
Machulla HJ, Marsmann M, Dutschka K (1980) Eur J Nucl Med 5:171–173
Knoop F (1905) Beitr Chem Physiol Path 6:150–153
Reske SN, Biersack H-J, Lackner K (1982) Nucl Med 6:249–253
Reske SN, Fuchs R, Machulla H-J, Winkler C (1982) Radiochem Radioanal Lett 55(4):257–264
Reske SN, Machulla H-J, Winkler C (1982) J Nucl Med 23(5):10
Reske SN, Simon H, Machulla H-J, Biersack H-J, Knopp R, Winkler C (1982) J Nucl Med 23(5):34
Reske SN, Sauer W, Machulla H-J, Knust J, Winkler C (1984) J Nucl Med 25:1335–1342
Reske SN, Schön S, Schmitt W, Machulla H-J, Knopp R, Winkler C (1986) Eur J Nucl Med 12:27–31
Bjorkman M, Andersson Y, Doi H, Kato K, Suzoki M, Noyori R, Watanabe Y, Långström B (1998) Acta Chem Scand 52:635–640
Hosoya T, Wakao M, Kondo Y, Doi H, Suzuki M (2004) Org Bio Mol Chem 2:24–27
Suzuki M, Doi H, Kato K, Bjorkman M, Långström B, Watanabed Y, Noyori R (2000) Tetrahedron 56:8263–8273
Karimi F, Barletta J, Långström B (2005) Eur J Org Chem 45:2374–2378
Hosoya T, Sumi K, Doi H, Wakaoa M, Suzuki M (2006) Org Biomol Chem 4:410–415
Miller PW, Long NJ, Vilar R, Gee AD (2008) Angew Chem 47:8998–9033
Al-Momani E, Zlatopolskiy BD, Solbach C, Reske SN, Machulla H-J (2010) J Radioanal Nucl Chem 286:231–234
Brunnjer RK, Broschberg H-J (1983) Helv Chem Acta 66:2608–2614
Cryle MJ, Matovic NJ, De Voss JJ (2003) Org Lett 5:3341–3344
Krapcho AP, Lovery AJ (1973) Tetrahedron Lett 12:957–960
Samuelsson L, Långström B (2003) J Label Compd Radiopharm 46:263–272
Acknowledgment
The authors would like to thank DAAD (Deutscher Akademischer Austauschdienst) for supporting this work by a research grant (A/07/80765).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Momani, E., Zlatopolskiy, B.D., Solbach, C. et al. Synthesis of 15-(4-[11C]methylphenyl)pentadecanoic acid (MePPA) via Stille cross-coupling reaction. J Radioanal Nucl Chem 288, 881–886 (2011). https://doi.org/10.1007/s10967-011-1022-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1022-1