Abstract
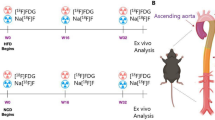
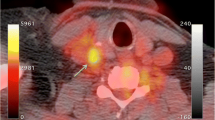
We investigated the ability of fluorodeoxyglucose positron emission tomography (FDG PET) imaging to serially monitor macrophage content in a rabbit model of atherosclerosis. Atherosclerosis was induced in rabbits (n = 8) by a combination of atherogenic diet and balloon denudation of the aorta. At the end of nine months, the rabbits were randomized to a further six months of the same atherogenic diet (progression group) or normal diet (regression group). In vivo uptake of FDG by the thoracic aorta was measured using aortic uptake-to-blood radioactivity ratios at the start and end of the randomized period. A significant increase in FDG uptake of the progression group after continued cholesterol feeding (aortic uptake-to-blood radioactivity: 0.57 ± 0.02 to 0.68 ± 0.02, P = 0.001), and a corresponding fall in FDG uptake of the regression group after returning to a normal chow diet (aortic uptake-to-blood radioactivity ratios: 0.67 ± 0.02 to 0.53 ± 0.02, P < 0.0001). FDG PET can quantify in vivo macrophage content and serially monitor changes in FDG activity in this rabbit model.





Similar content being viewed by others
References
Worthley SG, Helft G, Zaman AG et al (2000) Atherosclerosis and the vulnerable plaque–pathogenesis: part I. Aust N Z J Med 30:600–607
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126. doi:10.1056/NEJM199901143400207
Gronholdt ML, Dalager-Pedersen S, Falk E (1998) Coronary atherosclerosis: determinants of plaque rupture. Eur Heart J 19(Suppl C):C24–C29
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92:657–671
Libby P (1995) Molecular bases of the acute coronary syndromes. Circulation 91:2844–2850
Berliner J, Navab M, Fogelman A et al (1995) Atherosclerosis: basic mechanisms: oxidation, inflammation, and genetics. Circulation 91:2488–2496
Shah PK, Falk E, Badimon JJ et al (1995) Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 92:1565–1569
Galis ZS, Muszynski M, Sukhova GK et al (1994) Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res 75:181–189
Lendon C, Davies M, Born G et al (1991) Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 87:87–90. doi:10.1016/0021-9150(91)90235-U
Kubota R, Kubota K, Yamada S et al (1994) Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med 35:104–112
Som P, Atkins HL, Bandoypadhyay D et al (1980) A fluorinated glucose analog, 2-fluoro-2-deoxy-d-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med 21:670–675
Ogawa M, Ishino S, Mukai T et al (2004) (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 45:1245–1250
Rudd JH, Warburton EA, Fryer TD et al (2002) Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105:2708–2711. doi:10.1161/01.CIR.0000020548.60110.76
Zhang Z, Machac J, Helft G et al (2006) Non-invasive imaging of atherosclerotic plaque macrophage in a rabbit model with F-18 FDG PET: a histopathological correlation. BMC Nucl Med 6:3. doi:10.1186/1471-2385-6-3
Tahara N, Kai H, Ishibashi M et al (2006) Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 48:1825–1831. doi:10.1016/j.jacc.2006.03.069
Helft G, Worthley SG, Fuster V et al (2001) Atherosclerotic aortic component quantification by noninvasive magnetic resonance imaging: an in vivo study in rabbits. J Am Coll Cardiol 37:1149–1154. doi:10.1016/S0735-1097(01)01141-X
Helft G, Worthley SG, Fuster V et al (2002) Progression and regression of atherosclerotic lesions: monitoring with serial non-invasive magnetic resonance imaging. Circulation 105:993–998. doi:10.1161/hc0802.104325
Worthley SG, Helft G, Fuster V et al (2000) Serial in vivo MRI documents arterial remodelling in experimental atherosclerosis. Circulation 101:586–589
Vallabhajosula S, Fuster V (1997) Atherosclerosis: imaging techniques and the evolving role of nuclear medicine. J Nucl Med 38:1788–1796
Lees RS, Lees AM, Strauss HW (1983) External imaging of human atherosclerosis. J Nucl Med 24:154–156
Lees AM, Lees RS, Schoen FJ et al (1988) Imaging human atherosclerosis with 99mTc-labeled low density lipoproteins. Arteriosclerosis 8:461–470
Virgolani I (1989) Radiolabeling autologous monocytes with 111In oxine for reinjection im patients with atherosclerosis. Prog Clin Biol Res 355:271–274
Narula J, Petrov A, Bianchi C et al (1995) Noninvasive localization of experimental atherosclerotic lesions with mouse/human chimeric Z2D3 F(ab′)2 specific for the proliferating smooth muscle cells of human atheroma. Imaging with conventional and negative charge-modified antibody fragments. Circulation 92:474–484
Minar E, Ehringer H, Dudczak R et al (1989) Indium-111-labeled platelet scintigraphy in carotid atherosclerosis. Stroke 20:27–33
Davis HH, Siegel BA, Sherman LA et al (1980) Scintigraphic detection of carotid atherosclerosis with indium-111-labeled autologous platelets. Circulation 61:982–988
Mettinger KL, Larsson S, Ericson K et al (1978) Detection of atherosclerotic plaques in carotid arteries by the use of 123I-fibrinogen. Lancet 1:242–244. doi:10.1016/S0140-6736(78)90485-3
Fuster V, Badimon L, Badimon J et al (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes. N Eng J Med 326(242–250):310–318
van der Wal AC, Becker AE, van der Loos CM et al (1994) Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89:36–44
Raffel OC, Tearney GJ, Gauthier DD et al (2007) Relationship between a systemic inflammatory marker, plaque inflammation, and plaque characteristics determined by intravascular optical coherence tomography. Arterioscler Thromb Vasc Biol 27:1820–1827. doi:10.1161/ATVBAHA.107.145987
MacNeill BD, Jang IK, Bouma BE et al (2004) Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol 44:972–979. doi:10.1016/j.jacc.2004.05.066
Brezinski M, Tearney G, Nalwalk JW et al (1997) Assessing atherosclerotic plaque morphology: comparison of optical coherence tomography and high frequency intravascular ultrasound. Heart 77:397–403
Casscells W, Hathorn B, David M et al (1996) Thermal detection of cellular infiltrates in living atherosclerotic plaques: possible implications for plaque rupture and thrombosis. Lancet 347:1447–1451
Worthley SG, Helft G, Fuster V et al (2000) High resolution ex vivo magnetic resonance imaging of in situ coronary and aortic atherosclerotic plaque in a porcine model. Atherosclerosis 150:321–329. doi:10.1016/S0021-9150(99)00386-X
Toussaint J, LaMuraglia G, Southern J et al (1996) Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 94:932–938
Demacker PN, Dormans TP, Koenders EB et al (1993) Evaluation of indium-111-polyclonal immunoglobulin G to quantitate atherosclerosis in Watanabe heritable hyperlipidemic rabbits with scintigraphy: effect of age and treatment with antioxidants or ethinylestradiol. J Nucl Med 34:1316–1321
Di Chiro G, DeLaPaz RL, Brooks RA et al (1982) Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology 32:1323–1329
Weber G (1977) Enzymology of cancer cells (second of two parts). N Engl J Med 296:541–551
Renner ED, Plagemann PG, Bernlohr RW (1972) Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem 247:5765–5776
Acknowledgments
This work was supported by grants from the National Heart Foundation of Australia (SA Branch) (S.G.W.), the French Federation of Cardiology (G.H.) and the National Health and Medical Research Council of Australia (G.Y.H.L., No: 497809).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Worthley, S.G., Zhang, Z.Y., Machac, J. et al. In vivo non-invasive serial monitoring of FDG-PET progression and regression in a rabbit model of atherosclerosis. Int J Cardiovasc Imaging 25, 251–257 (2009). https://doi.org/10.1007/s10554-008-9377-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-008-9377-2