Abstract
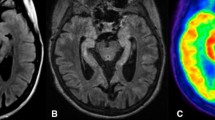
In parallel to the detection of new neuronal autoantibodies, the diagnosis of non-infectious limbic encephalitis has risen. Given that cerebral imaging studies show highly variable results, the present retrospective study investigates imaging findings in association with autoantibody type. An institutional database search identified 18 patients with non-infectious limbic encephalitis who had undergone [18F] fluorodeoxyglucose positron emission tomography (FDG-PET). Sixteen of these patients also underwent magnetic resonance imaging (MRI). MRI and FDG-PET images were categorized as follows: normal (0); mesiotemporal abnormality (1); normal mesiotemporal finding but otherwise abnormal (2). Neuronal autoantibodies were determined in serum and/or CSF. Autoantibodies were grouped according to the cellular localization of their target antigen: antibodies against surface antibodies (i.e., VGKC, NMDAR): 9; antibodies against intracellular antigens (i.e., Hu, Ri, GAD): 4; no autoantibodies: 5. The fraction of abnormal scans was lower for MRI (10/16) than for FDG-PET (14/18). There was a significant association between PET findings and autoantibody type: All patients with autoantibodies against intracellular antigens showed mesiotemporal findings on FDG-PET. In turn, only 2/9 patients with autoantibodies against surface antigens displayed mesiotemporal hypermetabolism. In the remaining seven patients, four scans were rated as normal and three only showed findings outside the mesiotemporal region. A similar association was found using MRI, although this did not reach statistical significance. Autoantibody type was found to be associated with FDG-PET and, to a lesser extent, with MRI imaging results. Our observations may explain the heterogeneity of imaging data in LE and based on in vivo findings support the assumption of different patho mechanisms underlying LE due to antibodies against surface and intracellular antigens, respectively.



Similar content being viewed by others
References
Scheid R, Lincke T, Voltz R et al (2004) Serial 18F-fluoro-2-deoxy-d-glucose positron emission tomography and magnetic resonance imaging of paraneoplastic limbic encephalitis. Arch Neurol 61:1785–1789
Provenzale JM, Barboriak DP, Coleman RE (1998) Limbic encephalitis: comparison of FDG-PET and MR imaging findings. Am J Roentgenol 170:1659–1660
Hoffmann LA, Jarius S, Pellkofer HL et al (2008) Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J Neurol Neurosurg Psychia 79:767–773
Greiner H, Leach JL, Lee KH et al (2011) Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure 20:266–270
Pillai SC, Gill D, Webster R et al (2010) Cortical hypometabolism demonstrated by PET in relapsing NMDA receptor encephalitis. Pediatr Neurol 43:217–220
Prüss H, Dalmau J, Harms L et al (2010) Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 75:1735–1739
Ishiura H, Matsuda S, Higashihara M et al (2008) Response of anti-NMDA receptor encephalitis without tumour to immunotherapy including rituximab. Neurology 71:1921–1923
Vitaliani R, Mason W, Ances B et al (2005) Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol 58:594–604
Gultekin SH, Rosenfeld MR, Voltz R et al (2000) Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 123:1481–1494
Dalmau J, Tüzün E, Wu HY et al (2007) Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 61:25–36
Maqbool M, Oleske DA, Huq AH et al (2011) Novel FDG-PET findings in NMDA receptor encephalitis: a case based report. Child Neurol 26:1325–1328
Caballero PE (2011) Fluorodeoxyglucose positron emission tomography findings in NMDA receptor antibody encephalitis. Arq Neuropsiquiatr 69:409–410
Padma S, Sundaram PS, Marmattom BV (2011) PET/CT in the evaluation of anti-NMDA-receptor encephalitis: what we need to know as a NM physician. Ind J Nucl Med 26:99–101
Haberlandt E, Bast T, Ebner A et al (2011) Limbic encephalitis in children and adolescents. Arch Dis Child 96:186–191
Maeder-Ingvar M, Prior JO, Irani SR et al (2011) FDG-PET hyperactivity in basal ganglia correlating with clinical course in anti-NDMA-R antibodies encephalitis. J Neurol Neurosurg Psychia 82:235–236
Guenther A, Brodoehl S, Witte OW et al (2012) Atypical posthypoxic MRI changes in hypermetabolic regions in anti-NMDA-receptor encephalitis. Neurology 79:720–721
Irani SR, Bera K, Waters P et al (2010) N-methyl-d-aspartate antibody encephalitis: temporal progression of clinical and para clinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133:1655–1667
Chatzikonstantinou A, Szabo K, Ottomeyer C et al (2009) Successive affection of bilateral temporomesial structures in a case of non-paraneoplastic limbic encephalitis demonstrated by serial MRI and FDG-PET. J Neurol 256:1753–1755
Troester F, Weske G, Schlaudraff E et al (2009) Image of the month. FDG-PET in paraneoplastic limbic encephalitis. Eur J Nucl Med Mol Imag 36:539
Ances BM, Vitaliani R, Taylor RA et al (2005) Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 128:1764–1777
Kassubek J, Juengling FD, Nitzsche EU et al (2001) Limbic encephalitis investigated by 18 FDG-PET and 3D MRI. J Neuroimag 11:55–59
Mohr BC, Minoshima S (2010) F-18 fluorodeoxyglucose PET/CT findings in a case of anti-NMDA receptor encephalitis. Clin Nucl Med 35:461–463
Leypoldt F, Buchert R, Kleiter I et al (2012) Fluorodeoxyglucose positron emission tomography in anti-N-methyl-d-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychia 83:681–686
Rey C, Koric L, Guedj E et al (2012) Striatal hypermetabolism in limbic encephalitis. J Neurol 259:1106–1110
Malter MP, Helmstaedter C, Urbach H et al (2010) Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 67:470–478
Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W et al (2004) Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychia 75:1135–1140
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M et al (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lan Neurol 7:1091–1098
Vincent A, Bien CG, Irani SR, Waters P (2011) Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lan Neurol 10:759–772
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE (1995) A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 36:1238–1248
Hellwig S, Amtage F, Kreft A, Buchert R, Winz OH, Vach W et al (2012) [18F]FDG-PET is superior to [123I]IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology 79:1314–1322
Blanc F, Ruppert E, Kleitz C, Valenti MP, Cretin B, Humbel RL et al (2009) Acute limbic encephalitis and glutamic acid decarboxylase antibodies: a reality? J Neurol Sci 287:69–71
Fisher RE, Patel NR, Lai EC, Schulz PE (2012) Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med 37:213–218
Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F et al (2012) Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135:1622–1638
Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R et al (2010) Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 30:5866–5875
Pellkofer HL, Kuempfel T, Jacobson L, Vincent A, Derfuss T (2010) Non-paraneoplastic limbic encephalitis associated with NMDAR and VGKC antibodies. J Neurol Neurosurg Psychia 81:1407–1408
Acknowledgments
The authors thank Dr. Sandra Dieni for helpful comments on the text.
Conflicts of interest
A.B and S.R. report receiving consulting and lecture fees, grant and research support from Bayer Vital GmbH, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis and Teva. S.R. is a founding member of ravo Diagnostika GmbH, Freiburg. I.M. is supported by the German Research Council (DFG MA-2343/41) and by the Bernstein Focus of Neuro technology (B3). P.T.M. received research grants from GE Healthcare and payments for lectures by Siemens AG and consultancy by Bayer-Schering AG. None of the authors has any financial or personal relationships with individuals or organizations that could inappropriately influence this submission.
Ethical standard
This study was approved by the local ethic committee and patients gave written informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baumgartner, A., Rauer, S., Mader, I. et al. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 260, 2744–2753 (2013). https://doi.org/10.1007/s00415-013-7048-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7048-2