Abstract
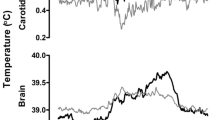
Direct measurements of deep-brain and body-core temperature were performed on rats to determine the influence of cerebral blood flow (CBF) on brain temperature regulation under static and dynamic conditions. Static changes of CBF were achieved using different anesthetics (chloral hydrate, CH; α-chloralose, αCS; and isoflurane, IF) with αCS causing larger decreases in CBF than CH and IF; dynamic changes were achieved by inducing transient hypercapnia (5% CO2 in 40% O2 and 55% N2). Initial deep-brain/body-core temperature differentials were anesthetic-type dependent with the largest differential observed with rats under αCS anesthesia (ca. 2°C). Hypercapnia induction raised rat brain temperature under all three anesthesia regimes, but by different anesthetic-dependent amounts correlated with the initial differentials—αCS anesthesia resulted in the largest brain temperature increase (0.32 ± 0.08°C), while CH and IF anesthesia lead to smaller increases (0.12 ± 0.03 and 0.16 ± 0.05°C, respectively). The characteristic temperature transition time for the hypercapnia-induced temperature increase was 2–3 min under CH and IF anesthesia and ~4 min under αCS anesthesia. We conclude that both, the deep-brain/body-core temperature differential and the characteristic temperature transition time correlate with CBF: a lower CBF promotes higher deep-brain/body-core temperature differentials and, upon hypercapnia challenge, longer characteristic transition times to increased temperatures.






Similar content being viewed by others
Abbreviations
- OEF:
-
Oxygen extraction fraction
- CBF:
-
Cerebral blood flow
- CH:
-
Chloral hydrate
- IF:
-
Isoflurane
- αCS:
-
α-Chloralose
- MABP:
-
Mean arterial blood pressure
- FiO2 :
-
Fraction of inspired oxygen
- CMRO2 :
-
Cerebral metabolic rate of oxygen
References
Artru AA, Michenfelder JD (1980) Effects of hypercarbia on canine cerebral metabolism and blood flow with simultaneous direct and indirect measurement of blood flow. Anesthesiology 52:466–469
Baker MA, Hayward JN (1968) The influence of the nasal mucosa and the carotid rete upon hypothalamic temperature in sheep. J Physiol 198:561–579
Berntman L, Dahlgren N, Siesjo BK (1979) Cerebral blood flow and oxygen consumption in the rat brain during extreme hypercarbia. Anesthesiology 50:299–305
Collins CM, Smith MB, Turner R (2004) Model of local temperature changes in brain upon functional activation. J Appl Physiol 97:2051–2055
Daniel PM, Dawes JDK, Prichard MML (1953) Studies of the carotid rete and its associated arteries. Philos Trans R Soc Lond B Biol Sci 237:173–208
Davis TL, Kwong KK, Weisskoff RM, Rosen BR (1998) Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA 95:1834–1839
Erickson KM, Lanier WL (2003) Anesthetic technique influences brain temperature, independently of core temperature, during craniotomy in cats. Anesth Analg 96:1460–1466
Gorbach AM (1993) Infrared imaging of brain function. Adv Exp Med Biol 333:95–123
Greeley WJ, Kern FH, Meliones JN, Ungerleider RM (1993) Effect of deep hypothermia and circulatory arrest on cerebral blood flow and metabolism. Ann Thorac Surg 56:1464–1466
Hayward JN, Baker MA (1968) Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol 215:389–403
Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB (1999) Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 42:849–863
Horvath I, Sandor NT, Ruttner Z, McLaughlin AC (1994) Role of nitric oxide in regulating cerebrocortical oxygen consumption and blood flow during hypercapnia. J Cereb Blood Flow Metab 14:503–509
Kassell NF, Hitchon PW, Gerk MK, Sokoll MD, Hill TR (1981) Influence of changes in arterial pCO2 on cerebral blood flow and metabolism during high-dose barbiturate therapy in dogs. J Neurosurg 54:615–619
Katz-Brull R, Alsop DC, Marquis RP, Lenkinski RE (2006) Limits on activation-induced temperature and metabolic changes in the human primary visual cortex. Magn Reson Med 56:348–355
Kauppinen RA, Vidyasagar R, Childs C, Balanos GM, Hiltunen Y (2008) Assessment of human brain temperature by 1H MRS during visual stimulation and hypercapnia. NMR Biomed 21:388–395
Kety SS, Schmidt CF (1947) The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27:484–492
Kim SG, Rostrup E, Larsson HB, Ogawa S, Paulson OB (1999) Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med 41:1152–1161
Kiyatkin EA, Brown PL, Wise RA (2002) Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci 16:164–168
Kliefoth AB, Grubb RL Jr, Raichle ME (1979) Depression of cerebral oxygen utilization by hypercapnia in the rhesus monkey. J Neurochem 32:661–663
LaManna JC, McCracken KA, Patil M, Prohaska O (1988) Brain tissue temperature: activation-induced changes determined with a new multisensor probe. Adv Exp Med Biol 222:383–389
Lindauer U, Villringer A, Dirnagl U (1993) Characterization of CBF response to somatosensory stimulation: model and influence of anesthetics. Am J Physiol 264:H1223–H1228
Lust A, Fuller A, Maloney SK, Mitchell D, Mitchell G (2007) Thermoregulation in pronghorn antelope (Antilocapra americana Ord) in the summer. J Exp Biol 210:2444–2452
Maloney SK, Mitchell D, Blache D (2007) The contribution of carotid rete variability to brain temperature variability in sheep in a thermoneutral environment. Am J Physiol Regul Integr Comp Physiol 292:R1298–R1305
Mariak Z (2002) Intracranial temperature recordings in human subjects. The contribution of the neurosurgeon to thermal physiology. J Therm Biol 27:219–228
McElligott JG, Melzack R (1967) Localized thermal changes evoked in the brain by visual and auditory stimulation. Exp Neurol 17:293–312
Mellergard P (1995) Intracerebral temperature in neurosurgical patients: intracerebral temperature gradients and relationships to consciousness level. Surg Neurol 43:91–95
Melzack R, Casey KL (1967) Localized temperature changes evoked in the brain by somatic stimulation. Exp Neurol 17:276–292
Michenfelder JD, Milde JH (1991) The relationship among canine brain temperature, metabolism, and function during hypothermia. Anesthesiology 75:130–136
Mitchell G, Fuller A, Maloney SK, Rump N, Mitchell D (2006) Guttural pouches, brain temperature and exercise in horses. Biol Lett 2:475–477
Miyazawa T, Hossmann KA (1992) Methodological requirements for accurate measurements of brain and body temperature during global forebrain ischemia of rat. J Cereb Blood Flow Metab 12:817–822
Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L (2001) Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci USA 98:7593–7598
Neimark MA, Konstas AA, Laine AF, Pile-Spellman J (2007) Integration of jugular venous return and circle of Willis in a theoretical human model of selective brain cooling. J Appl Physiol 103:1837–1847
Neimark MA, Konstas AA, Choi JH, Laine AF, Pile-Spellman J (2008) Brain cooling maintenance with cooling cap following induction with intracarotid cold saline infusion: a quantitative model. J Theor Biol 253:333–344
Nelson DA, Nunneley SA (1998) Brain temperature and limits on transcranial cooling in humans: quantitative modeling results. Eur J Appl Physiol Occup Physiol 78:353–359
Nybo L (2007) Exercise and heat stress: cerebral challenges and consequences. Prog Brain Res 162:29–43
Nybo L, Secher NH (2004) Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol 72:223–261
Ogawa S, Lee T, Nayak AS, Glynn P (1990) Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high fields. Magn Reson Med 14:68–78
Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K (1993) Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 64:803–812
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682
Sedunova EV (1992) Brain temperature in small birds and mammals. Fiziol Zh SSSR Im I M Sechenova 78:85–89
Shevelev IA (1998) Functional imaging of the brain by infrared radiation (thermoencephaloscopy). Prog Neurobiol 56:269–305
Siesjo B (1978) Brain energy metabolism. Wiley, New York
Sukstanskii AL, Yablonskiy DA (2004) An analytical model of temperature regulation in human head. J Therm Biol 29:583–587
Sukstanskii AL, Yablonskiy DA (2006) Theoretical model of temperature regulation in the brain during changes in functional activity. Proc Natl Acad Sci USA 103:12144–12149
Swan H (1974) Thermoregulation and bioenergetics. American Elsevier Publishing Company, New York
Trubel HK, Sacolick LI, Hyder F (2006) Regional temperature changes in the brain during somatosensory stimulation. J Cereb Blood Flow Metab 26:68–78
Yablonskiy DA, Ackerman JJ, Raichle ME (2000) Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci USA 97:7603–7608
Yang SP, Krasney JA (1995) Cerebral blood flow and metabolic responses to sustained hypercapnia in awake sheep. J Cereb Blood Flow Metab 15:115–123
Young WL, Prohovnik I, Correll JW, Ostapkovich N, Ornstein E, Quest DO (1991) A comparison of cerebral blood flow reactivity to CO2 during halothane versus isoflurane anesthesia for carotid endarterectomy. Anesth Analg 73:416–421
Zhu M, Nehra D, Ackerman JJ, Yablonskiy DA (2004) On the role of anesthesia on the body/brain temperature differential in rats. J Therm Biol 29:599–603
Zhu M, Ackerman JJ, Sukstanskii AL, Yablonskiy DA (2006) How the body controls brain temperature: the temperature shielding effect of cerebral blood flow. J Appl Physiol 101:1481–1488
Acknowledgments
We would like to thank Dr. Sukstanskii for helpful discussion. This study was supported by NIH Grants RO1-NS41519 and R24-CA83060 (Small Animal Imaging Resource Program)
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Zhu, M., Ackerman, J.J.H. & Yablonskiy, D.A. Body and brain temperature coupling: the critical role of cerebral blood flow. J Comp Physiol B 179, 701–710 (2009). https://doi.org/10.1007/s00360-009-0352-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-009-0352-6