Abstract
Gastrin releasing peptide (GRP) is a neuropeptide that has been suggested to play a role in the development of some malignancies. Our aim was: (1) to identify the expression of GRP in cancerous prostate glands, and (2) to correlate its expression to various pathological parameters and to the patient's clinical outcome. Using standard immunohistochemistry, we evaluated GRP expression in both biopsy and radical prostatectomy specimens from 30 patients with prostatic adenocarcinomas. GRP was expressed in 18 radical prostatectomy specimens (60%) and in 15 biopsies (50%). There was an association between positive immunoexpression of GRP, relapse (P=0.029) and advanced tumor stages (i.e. pT3, pT4) (P=0.049). In the respective biopsies, GRP immunostatus was similar to that observed in the subsequent radical prostatectomy specimens. GRP immunoexpression may be of some value as a diagnostic and prognostic marker. Patients whose pathology specimens demonstrate GRP immunopositivity should be closely monitored, since they appear to be at higher risk of disease progression and relapse.
Similar content being viewed by others
Introduction
Prostate cancer is the most common non-cutaneous malignant tumor in men. At present, modalities for the treatment of advanced prostate cancer are palliative and most patients can be treated by androgen suppression or androgen receptor blockade [6]. However, a major problem in the treatment of metastatic prostate cancer is the development of androgen-independent disease. This androgen-independent growth can be caused by several mechanisms [3], one of which is the receptor-specific, paracrine or autocrine, growth modulation of human prostatic cancers by neuropeptides secreted by neuroendocrine (NE) cells [8].
NE cells are present in both the normal and neoplastic prostate [13]. Neuropeptides are growth factors that are produced by these cells, either eutopically or ectopically. In the prostate gland, a number of neuropeptides have been found to be present both in NE cells and in autonomic and sensory nerve terminals [5]. These include gastrin releasing peptide (GRP), calcitonin gene-related peptide (CGRP), substance P), neuropeptide Y, vasoactive intestinal polypeptide (VIP), parathyroid hormone-related protein (PTH-rP) and calcitonin [5]. The role of NE cells and their secreted bioactive neuropeptides in the progression of human prostate cancer from an androgen-dependent to an androgen-independent state is still controversial [8, 12]. Neuroendocrine differentiation occurs with higher frequency in prostate cancer than in other carcinomas of the genitourinary tract and has been correlated with tumor progression and the androgen-independent state [8].
However, only little is known about the effect of neuropeptides on tumor invasion [10]. GRP, CGRP, VIP and PTH-rP increase the invasive potential of prostate cancer cells, partially through enhancement of cell motility [10, 11]. Furthermore, it has been proposed that the secretion of GRP by NE cells might be responsible not only for prostate cancer progression but also for androgen independence and a poor prognosis [1]. Recently, peptide analogues have been suggested as an additional treatment for advanced prostate cancer [16].
The aim of the present study is: (1) to identify, using standard immunohistochemistry, the expression of the GRP neuropeptide in cancerous prostate glands, and (2) to correlate this expression to various pathological parameters (e.g. Gleason score) and to clinical outcome.
Materials and methods
Thirty human, histologically conventional, prostate adenocarcinomas from radical prostatectomies were obtained from patients 57–66 years of age (mean age: 65.4 years) at the time of initial surgical treatment. In addition, we obtained and examined the respective transrectal, ultrasound guided, prostate biopsy specimens of these patients prior to surgery. As normal controls, we evaluated ten normal human prostate glands taken from autopsies of motor accident victims (range: 22–36 years), and ten hyperplastic but otherwise normal prostate glands taken from patients having undergone transurethral prostatectomy (range of age: 51–69 years). The local ethics committee approved the collection and use of these specimens for our study.
Prior to diagnosis, our patients had either an elevated serum prostate specific antigen (PSA) level (>4 ng/ml) and/or a digital rectal examination that revealed a suspicious area in the prostate gland. In patients with PSA values >10 ng/ml, a free to total PSA ratio was obtained. Biopsies were performed in cases where the ratio was less than 0.2. In all of our patients, an ultrasound guided transrectal biopsy of the prostate was performed by the same experienced urologist or under his direct supervision. All 30 patients who were diagnosed with prostate adenocarcinomas underwent radical prostatectomy, maintaining the neurovascular bundle whenever possible. The preoperative tumor stage of our patients was not greater than T2b N0 M0 based on the 1997 TNM classification. Furthermore, only patients whose PSA values fell under 0.2 ng/ml after surgery were included. Preoperative evaluation consisted of an abdominal CT scan as well as a bone scan to exclude any metastatic disease. Specimens derived from radical prostatectomies were staged according to the 1997 TNM classification and graded according to Gleason score (Table 1). Pathological evaluation included tumor location, multifocality, perineural invasion and inspection of the surgical margins.
We evaluated all specimens, including biopsy material, for GRP immunoexpression using semi-quantitative methods after microscopic evaluation. Specimens were fixed in buffered formalin for no more than 24 hours. A standard, three-step, streptAB complex/HRP staining procedure (Dako, Glostrup, Denmark), for paraffin-embedded 4 μm-thick tissue sections was applied. A rabbit anti-human GRP antibody (at a concentration of 5.0 g/l, Dako A 0429) labeling GRP-producing cells in normal and neoplastic tissue, was used at a dilution of 1:50 for 30 min at room temperature. Alternative sections from the same paraffin blocks were also processed in each experiment without the application of the primary antibody to exclude non specific staining.
Out of the 30 patients, six of the initial biopsies were tumor-negative. These patients were asked to return for a repeat biopsy 6 weeks later. The patients whose biopsies had a high GRP expression, despite lack of malignancy (n=2), were asked to have the repeat biopsy 3 weeks after the initial one. None of our patients required a third biopsy.
The mean follow-up time after surgery was 46.2 months. All patients were routinely evaluated for serum PSA every 3 months for the first and second years after surgery. After the second year, PSA measurements were only made every 6 months. A biological recurrence was considered present if serum PSA values increased over 0.2 ng/ml. If a recurrence was suspected, an abdominal CT scan and a bone scan were performed. These patients were then put on total androgen deprivation. Recurrent high levels of PSA, despite androgen deprivation, were considered compatible with the androgen independent stage of the disease.
Statistics
χ2-testsor Fisher's exact tests were employed to investigate the association between GRP expression and tumor stage, perineunal invasion and Gleason scores. The immunoexpression of GRP with regards to PSA levels and age was evaluated by t-tests. Disease-free survival curves were assessed by log-rank statistics.
Results
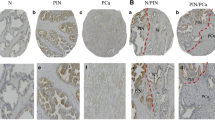
GRP immunoexpression demonstrated a diffuse cytoplasmic staining pattern. The marker was expressed in the cytoplasm of parenchymal tumor cells in ≥30% of cases (range 30–85%) (Fig. 1). GRP immunopositivity was observed in 18 out of 30 radical prostatectomy specimens (60%) and in 15 prostate biopsies obtained preoperatively from the same 30 patients (50%). In GRP-immunoreactive invasive carcinomas, GRP immunolabeling was evident in tumor adjacent PIN areas, apart from malignant cells, as well as in entrapped or neighboring hyperplastic glands (Fig. 2). In contrast, in non-cancerous prostatic tissue (normal controls), in which normal or hyperplastic glands were observed, a minimal degree of GRP immunostaining was detected in scattered cells within the glandular epithelium. In all cases, an occasional stromal immunoreactivity to GRP was detectable. Out of the 30 patients, six relapsed during follow-up; one of these patients has entered an androgen independent stage. All six patients were found to be GRP positive (P=0.029) (Fig. 3). Cases with locally advanced prostate cancer (i.e. pT3, pT4) were more often GRP-immunoreactive compared to those with earlier stages (i.e. pT1,pT2) (P=0.049) (Fig. 4). With regard to Gleason scores, GRP immunoreactivity was more frequent in cases with moderate or moderate–low differentiation (scores 5, 6 and 7 respectively). However, this did not reach significance (P=0.662). GRP immunostaining was not statistically linked with perineural invasion, presence of nodal involvement, or Gleason score pattern (4+3 versus 3+4) in Gleason score 7 tumors. As far as Gleason score 8 and 9 cases were concerned, no statistical conclusion could be drawn due to low sample size (n=2); however, these cases lacked GRP immunoexpression. Furthermore, there was no significant association between GRP immunoexpression and the preoperative serum PSA values, nor the free to total PSA ratio. Due to the relatively small number of relapsing patients included in this study, no relationship could be established between GRP immunoexpression and the development of androgen-independent disease.
In the biopsies prior to surgery, GRP immunostatus was similar to that observed in the subsequent radical prostatectomy specimens. In the initial biopsies which were negative for malignancy (n=6), GRP immunoreactivity could be observed in two patients. In these two patients, acini without any apparent morphology of malignancy or PIN grade were characterized by GRP positivity.
Discussion
Bombesin-like peptides are produced in various types of cancers [16]. Specific binding sites for GRP (the mammalian counterpart of bombesin) were detected in androgen-independent prostate cancer lines and in surgical specimens of prostate cancer [1, 9, 15, 17]. Bombesin-like peptides can stimulate the growth of PC-3 and DU-145 human, androgen-independent, prostate cancer cell lines [2], promote the invasiveness of DU-145 cells in vitro [7] and facilitate the invasion of both PC-3 and DU-145 cells to Matrigel (a reconstituted basement membrane) [10, 11]. The secretion of bombesin/GRP has been suggested to be responsible for the progression, androgen independence and poor prognosis of prostate cancer [1].
The findings that GRP can act as an autocrine growth factor for small cell lung carcinoma and other tumors have encouraged several laboratories to synthesize GRP receptor antagonists for the hormonal treatment of these malignancies. Bombesin/GRP antagonists inhibit the growth of prostate cancer cell lines PC-3 and DU-145 and are undergoing clinical trials [16]. Moreover, recent studies have shown that bombesin/GRP antagonists can augment the inhibitory effects of GH-RH antagonists on the growth of PC-3 prostate cancers [14]. Efforts are being made to establish GRP presence both in localized prostate cancer and in bone metastases using scintigraphy [4].
In this study, we demonstrated the immunoexpression of GRP in a considerable number of conventional prostate adenocarcinomas and we tried to determine the correlation between the expression of this molecule and the clinical outcome in patients. To the best of our knowledge, this is the first time that the expression of GRP in prostate cancer has been confirmed using immunohistochemical methods. We examined both biopsy specimens and the respective pathology specimens deriving from radical prostatectomies.
In the specimens examined, GRP immunopositivity status was mainly observed in tumors of moderate differentiation, while poorly differentiated carcinomas lacked GRP immunoexpression. This observation is in accordance with the postulated progressive reduction in neuroendocrine differentiation with advancing tumor grade [19]. Furthermore, there appears to be a correlation of GRP immunoexpression and relapse after radical prostatectomy. All of our patients who relapsed were GRP-immunopositive. This could mean that GRP expression is related to the aggressiveness of the disease; from the clinical point of view, patients with tumors that are found to be GRP-immunopositive in pathology specimens seem to relapse more quickly than patients with GRP-immunonegative tumors. Additional evidence to support the aggressiveness of prostate cancer in patients who are GRP-immunopositive is the finding that there is a connection between the expression of GRP and the patient's tumor stage. Patients with either a pT3 or pT4 tumors were more frequently GRP-immunopositive than patients with earlier tumor stages.
The fact that GRP immunoexpression was observed in tumor-adjacent acini, either in radical prostatectomy specimens or in biopsies with subsequent cancer diagnosis, suggests that GRP immunoexpression is related to a paracrine, growth promoting activity. Our results are compatible with those of a recent study in which the relationship between GRP receptors and prostate neoplastic transformation was examined [9]. In that study, GRP receptors were predominantly located in malignant specimens. Similarly, we did not observe any significant GRP immunoreactivity in normal benign prostate glands, or in hyperplastic glands from tumor-negative controls. The minimal detection of GRP-immunopositive cells in normal controls can be justified by the occasional presence of neuroendocrine argentaffin cells within the prostate glandular epithelium [18]. The latter likely arise through differentiation of the prostate epithelium and probably exert a regulatory role on prostate growth and function through both neuroendocrine and paracrine mechanisms [18].
It is noteworthy that we found similar results on the distribution of GRP in pathology specimens following radical prostatectomies and in the respective biopsy specimens. However, there were cases in which a positive immunoexpression of GRP occurred in biopsies that had not initially been positive for cancer. According to our research protocol, these patients were asked to repeat the biopsy much sooner than the average period routinely recommended in our department, which is 6 weeks. Such patients were diagnosed with prostate carcinomas; this could imply that GRP immunoexpression is of diagnostic significance in prostate biopsies. Patients with elevated serum PSA values, no apparent cancer pathology in prostate biopsies, but who are GRP immunopositivity in prostatic glandular epithelia, should be kept under close observation with frequent measurements of serum PSA levels and repeat biopsies. However, changes in every day practice can not be recommended until our observations are confirmed in future studies. Since we commenced the study of this molecule, we routinely examine the immunohistochemical distribution of GRP in biopsies that are initially negative for cancer and we recommend that GRP-immunopositive patients have a repeat biopsy in 3 weeks (instead of 6 weeks) and measurement of PSA levels every 4 weeks. The results of biopsy material are still pending, since an adequate number of patients should be analysed before conclusions are drawn.
Based on our observations, there appears to be a link between prostate cancer development and GRP expression. Even though the number of patients that were included in the present study is relatively small, we think that it is adequate to draw some preliminary conclusions about the possible role of GRP as a diagnostic and prognostic indicator in prostate cancer. However, additional studies using a larger number of patients should be performed to verify our findings. The results of this study are preliminary since patients who underwent surgery over the last 2 years were not included. It is worth investigating whether there is any connection between GRP immunoexpression and the patients who eventually develop a hormone-independent stage of the disease. In our study, there was only one patient who entered this stage. The tumor specimen from this patient was GRP-immunopositive.
References
Aprikian AG, Han K, Guy L, Landry F, Begin LR, Chevalier S (1998) Neuroendocrine differentiation and the bombesin/gastrin-releasing peptide family of neuropeptides in the progression of human prostate cancer. Prostate (Suppl 8):52–61
Bologna M, Festuccia C, Muzi P, Biordi L, Ciomei M (1989) Bombesin stimulates growth of human prostatic cancer cells in vitro. Cancer 63:1714–1720
Culig Z, Hobisch A, Hittmair A, Cronauer MV, Radmayr C, Zhang J, Bartsch G, Klocker H (1997) Synergistic activation of androgen and luteinizing hormone-releasing hormone in prostatic carcinoma cells. Prostate 32:106–114
De Vincentis G, Scopinaro F, Varvarigou A, Ussof W, Schillaci O, Archimandritis S, Leontiadis L,Corleto V, Longo F, Delle Fave G (2002) Phase I trial of technetium [LEU 13] bombesin as a cancer seeking agent. Possible scintigraphic guide for surgery? Tumori 88:28–30
Gkonos PJ, Krongrad A, Roos BA (1995) Neuroendocrine peptides in the prostate. Urol Res 23:81–87
Hegarty NJ, Fitzpatrick JM, Richie JP, Scardino PT, deVere White RW, Schröder FH, Coffey DS (1999) Future prospects in prostate cancer. Prostate 40:261–268
Hoosein NM, Logothetis CJ, Chung LW (1993) Differential effects of peptide hormones bombesin, vasoactive intestinal polypeptide and somatostatin analogue RC160 on the invasive capacity of human prostatic carcinoma cells. J Urol 149:1209–1213
Jongsma J, Oomen MHA, Noordzij MA, Romijn JC, van der Kwast TH, Schröder, van Steenbrugge GJ (2000) Androgen-independent growth is induced by neuropeptides in human prostate cancer cell lines. Prostate 42:34–44
Markwalder R, Reubi JC (1999) Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res 59:1152–1159
Nagakawa O, Ogasawara M, Fujii H (1998) Effect of prostatic neuropeptides on invasion and migration of PC-3 prostate cancer cells. Cancer Lett 133:27–33
Nagakawa O, Ogasawara M, Murata J, Fuse H, Saiki I (2001) Effect of prostatic neuropeptides on migration of prostate cancer cell lines. Int J Urol 8:65–70
Nakopoulou L, Stefanaki K, Deliveliotis C, Lazaris AC, Kondothanasis D, Dimopoulos C (1996) Neuroendocrine differentiation and proliferation state estimation in the hyperplastic and neoplastic human prostate. J Urol Pathol 4:239–251
Noordji MA, van Steenbrugge GJ, van der Kwast T, Schröder FH (1995) Neuroendocrine cells in the normal, hyperplastic and neoplastic prostate. Urol Res 22:333–341
Plonowski A, Schally AV, Varga JL, Rekasi Z, Hebert F, Halmos G, Groot K (2000) Potentiation of the inhibitory effect of growth hormone-releasing hormone antagonists on PC-3 human prostate cancer by bombesin antagonists indicative of interference with both IGF and EGF pathways. Prostate 44:172–180
Reile H, Armatis PE, Schally AV (1994) Characterization of high affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: internalization of receptor bound125I-[Tyr4] bombesin by tumor cells. Prostate 25:29–38
Schally AV, Comaru-Schally AM, Plonowski A, Nagy A, Halmos G, Rekasi Z (2000) Peptide analogs in the therapy of prostate cancer. Prostate 45:158–166
Sun B, Halmos G, Schally AV, Wang X, Martinez M (2000) Presence of receptors for bombesin/gastrin releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate 42:295–303
Young RH, Srigley JR, Amin MB, Ulbright TM, Cubilla AL (2000) Tumors of the prostatic gland, seminal vesicles, male urethra and penis. In: Young RH, Srigley JR, Amin MB et al. (eds) Atlas of tumor pathology. AFIP, Washington DC, pp 1–30
Young RH, Srigley JR, Amin MB, Ulbright TM, Cubilla AL (2000) Carcinoma of the prostatic gland. In: Young RH et al. (eds) Atlas of tumor pathology, AFIP, Washington DC, pp 111–217
Acknowledgment
The authors wish to thank Miss Eftaxia Karadima, technician, for her valuable help at various technical aspects of the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Constantinides, C., Lazaris, A.C., Haritopoulos, K.N. et al. Immunohistochemical detection of gastrin releasing peptide in patients with prostate cancer. World J Urol 21, 183–187 (2003). https://doi.org/10.1007/s00345-003-0339-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-003-0339-y