Abstract
Objectives
To validate a visual rating scale of frontal atrophy with quantitative imaging and study its association with clinical status, APOE ε4, CSF biomarkers, and cognition.
Methods
The AddNeuroMed and ADNI cohorts were combined giving a total of 329 healthy controls, 421 mild cognitive impairment patients, and 286 Alzheimer’s disease (AD) patients. Thirty-four patients with frontotemporal dementia (FTD) were also included. Frontal atrophy was assessed with the frontal sub-scale of the global cortical atrophy scale (GCA-F) on T1-weighted images. Automated imaging markers of cortical volume, thickness, and surface area were evaluated. Manual tracing was also performed.
Results
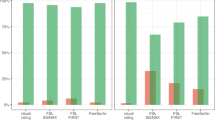
The GCA-F scale reliably reflects frontal atrophy, with orbitofrontal, dorsolateral, and motor cortices being the regions contributing most to the GCA-F ratings. GCA-F primarily reflects reductions in cortical volume and thickness, although it was able to detect reductions in surface area too. The scale showed significant associations with clinical status and cognition.
Conclusion
The GCA-F scale may have implications for clinical practice as supportive diagnostic tool for disorders demonstrating predominant frontal atrophy such as FTD and the executive presentation of AD. We believe that GCA-F is feasible for use in clinical routine for the radiological assessment of dementia and other disorders.
Key points
• The GCA-F visual rating scale reliably reflects frontal brain atrophy.
• Orbitofrontal, dorsolateral, and motor cortices are the most contributing regions.
• GCA-F shows significant associations with clinical status and cognition.
• GCA-F may be supportive diagnostic tool for disorders demonstrating predominant frontal atrophy.
• GCA-F may be feasible for use in radiological routine.




Similar content being viewed by others
Abbreviations
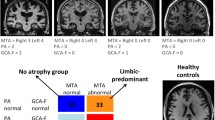
- MTA:
-
medial temporal atrophy
- PA:
-
posterior atrophy
- GCA:
-
global cortical atrophy
- GCA-F:
-
global cortical atrophy – frontal sub-scale
- SFG:
-
superior frontal gyrus
- MFG:
-
middle frontal gyrus
- IFG:
-
inferior frontal gyrus
- ORB:
-
orbitofrontal cortex
- DACC:
-
and dorsal anterior cingulate gyrus
References
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:263–269
Scheltens P, Leys D, Barkhof F et al (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55:967–972
Dubois B, Feldman HH, Jacova C et al (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746
Shim YS, Youn YC, Na DL et al (2011) Effects of medial temporal atrophy and white matter hyperintensities on the cognitive functions in patients with Alzheimer’s disease. Eur Neurol 66:75–82
Scheltens P, Fox N, Barkhof F, De Carli C (2002) Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol 1:13–21
Deweer B, Lehericy S, Pillon B et al (1995) Memory disorders in probable Alzheimer’s disease: the role of hippocampal atrophy as shown with MRI. J Neurol Neurosurg Psychiatry 58:590–597
Koedam EL, Lehmann M, van der Flier WM et al (2011) Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol 21:2618–2625
Lehmann M, Koedam EL, Barnes J et al (2012) Posterior cerebral atrophy in the absence of medial temporal lobe atrophy in pathologically-confirmed Alzheimer’s disease. Neurobiol Aging 33:627.e1–627.e12
Elliott R (2003) Executive functions and their disorders. Br Med Bull 65:49–59
Pasquier F, Leys D, Weerts JG, Mounier-Vehier F, Barkhof F, Scheltens P (1996) Inter-and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol 36:268–272
Scheltens P, Pasquier F, Weerts JG, Barkhof F, Leys D (1997) Qualitative assessment of cerebral atrophy on MRI: inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol 37:95–99
Henneman WJP, Vrenken H, Barnes J et al (2009) Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurology 73:935–940
Van der Vlies AE, Goos JDC, Barkhof F, Scheltens P, van der Flier WM (2012) Microbleeds do not affect rate of cognitive decline in Alzheimer disease. Neurology 79:763–769
Doody RS, Azher SN, Haykal HA, Dunn JK, Liao T, Schneider L (2000) Does APO 4 correlate with MRI changes in Alzheimer’s disease? J Neurol Neurosurg Psychiatry 69:668–671
Biessels GJ, De Leeuw F-E, Lindeboom J, Barkhof F, Scheltens P (2006) Increased cortical atrophy in patients with Alzheimer’s disease and type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 77:304–307
Goos JDC, Kester MI, Barkhof F et al (2009) Patients with Alzheimer disease with multiple microbleeds: relation with cerebrospinal fluid biomarkers and cognition. Stroke 40:3455–3460
Kim Y-S, Lee K-M, Choi BH, Sohn EH, Lee AY (2009) Relation between the clock drawing test (CDT) and structural changes of brain in dementia. Arch Gerontol Geriatr 48:218–221
Ferreira D, Cavallin L, Larsson E-M, et al. (2015) Practical cut-offs for visual rating scales of medial temporal, frontal, and posterior atrophy in Alzheimer’s disease and mild cognitive impairment. J Intern Med 278:277–290
Möller C, van der Flier WM, Versteeg A et al (2014) Quantitative regional validation of the visual rating scale for posterior cortical atrophy. Eur Radiol 24:397–404
Bresciani L, Rossi R, Testa C et al (2005) Visual assessment of medial temporal atrophy on MR films in Alzheimer’s disease: comparison with volumetry. Aging Clin Exp Res 17:8–13
Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR (2009) Development of an MRI rating scale for multiple brain regions: comparison with volumetrics and with voxel-based morphometry. Neuroradiology 51:491–503
Simmons A, Westman E, Muehlboeck S et al (2011) The AddNeuroMed framework for multi-centre MRI assessment of Alzheimer’s disease: experience from the first 24 months. Int J Geriatr Psychiatry 26:75–82
Mueller SG, Weiner MW, Thal LJ et al (2005) Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement 1:55–66
Petersen RC, Aisen PS, Beckett LA et al (2010) Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74:201–209
Lindberg O, Östberg P, Zandbelt BB, et al. (2009) Cortical morphometric subclassification of frontotemporal lobar degeneration. Am J Neuroradiol 30:1233–1239
Neary D, Snowden J, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Jack CR, Bernstein MA, Fox NC et al (2008) The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 27:685–691
Liu Y, Paajanen T, Zhang Y et al (2011) Combination analysis of neuropsychological tests and structural MRI measures in differentiating AD, MCI and control groups--the AddNeuroMed study. Neurobiol Aging 32:1198–1206
Kipps CM, Davies RR, Mitchell J, Kril JJ, Halliday GM, Hodges JR (2007) Clinical significance of lobar atrophy in frontotemporal dementia: application of an MRI visual rating scale. Dement Geriatr Cogn Disord 23:334–342
Victoroff J, Mack W, Grafton S, Schreiber SS, Chui HC (1994) A method to improve interrater reliability of visual inspection of brain MRI scans in dementia. Neurology 44:2267–2276
Ferreira D, Molina Y, Machado A et al (2014) Cognitive decline is mediated by gray matter changes during middle age. Neurobiol Aging 35:1086–1094
Dickerson BC, Feczko E, Augustinack JC et al (2009) Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging 30:432–440
Nygaard GO, Walhovd KB, Sowa P et al (2014) Cortical thickness and surface area relate to specific symptoms in early relapsing-remitting multiple sclerosis. Mult Scler 21:402–414
Jubault T, Gagnon J-F, Karama S et al (2011) Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage 55:462–467
Meda SA, Pryweller JR, Thornton-Wells TA (2012) Regional brain differences in cortical thickness, surface area and subcortical volume in individuals with Williams syndrome. PLoS One 7, e31913
Rimol LM, Nesvåg R, Hagler DJ et al (2012) Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry 71:552–560
Miller B, Chang L, Mena I, Boone K, Lesser IM (1993) Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia 4:204–213
Grossman M, McMillan C, Moore P et al (2004) What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain 127:628–649
Lindberg O, Manzouri A, Westman E, Wahlund LO (2012) A comparison between volumetric data generated by voxel-based morphometry and manual parcellation of multimodal regions of the frontal lobe. Am J Neuroradiol 33:1957–1963
Whitwell JL, Jack CR (2005) Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Top Magn Reson Imaging 16:409–425
Landis J, Koch G (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Crespo-Facorro B, Kim J, Andreasen NC et al (2000) Cerebral cortex: a topographic segmentation method using magnetic resonance imaging. Psychiatry Res 100:97–126
Dale AM, Sereno MI (1993) Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 5:162–176
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis i: segmentation and surface reconstruction. NeuroImage 9:179–194
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980
Eritaia J, Wood SJ, Stewart GW et al (2000) An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med 44:973–977
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage 9:195–207
Fischl B, Sereno MI, Tootell RB, Dale AM (1999) High-resolution inter-subject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055
Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20:70–80
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Fischl B, Salat DH, Van der Kouwe AJW et al (2004) Sequence-independent segmentation of magnetic resonance images. NeuroImage 23(Suppl 1):S69–S84
Fischl B, van der Kouwe A, Destrieux C et al (2004) Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22
Fornito A, Whittle S, Wood SJ, Velakoulis D, Pantelis C, Yücel M (2006) The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. NeuroImage 33:843–854
McCormick LM, Ziebell S, Nopoulos P, Cassell M, Andreasen NC, Brumm M (2006) Anterior cingulate cortex: an MRI-based parcellation method. NeuroImage 32:1167–1175
Reuter M, Rosas HD, Fischl B (2010) Highly accurate inverse consistent registration: a robust approach. NeuroImage 53:1181–1196
Ségonne F, Dale AM, Busa E et al (2004) A hybrid approach to the skull stripping problem in MRI. NeuroImage 22:1060–1075
Ségonne F, Pacheco J, Fischl B (2007) Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 26:518–529
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Acknowledgments
The scientific guarantor of this publication is Dr. Daniel Ferreira (Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Sweden). The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors thank Swedish Brain Power, the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro), The Swedish Alzheimer Foundation, and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council. AddNeuroMed is supported by InnoMed (Innovative Medicines in Europe), an Integrated Project funded by the European Union of the Sixth Framework programme priority FP6-2004-LIFESCIHEALTH-5, Life Sciences, Genomics and Biotechnology for Health. Data collection and sharing for the ADNI project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
One of the authors has significant statistical expertise. Institutional Review Board approval was not required because this study includes data from AddNeuroMed and ADNI, two public and available datasets for imaging research. Data collection was subject to ethical review and approval by committees from each participating centre. This study also includes data from the Memory Clinic at Karolinska University Hospital. This sample has been previously investigated and the study was approved by the Regional Ethical Review Board in Stockholm, Sweden. Written informed consent was not required for this study because this study includes data from AddNeuroMed and ADNI, two public and available datasets for imaging research. Data collection was subject to ethical review and approval by committees from each participating center. This study also includes data from the Memory Clinic at Karolinska University Hospital. This sample has been previously investigated and written informed consent was already available for all the subjects. Some study subjects or cohorts have been previously reported. Data from the AddNeuromed and the ADNI studies have been extensively used and reported. Given the amount of manuscript published using these two datasets, it is not possible to mention the number of patients previously published. This study also includes data from thirty-four patients previously investigated in the studies listed below.
- Looi et al. AJNR Am J Neuroradiol 2008
- Lindberg et al. AJNR Am J Neuroradiol 2009
- Looi et al. AJNR Am J Neuroradiol 2009
- Looi et al. Neuroimage 2010
- Looi et al. Psychiatry Res 2011
- Lindberg et al. J Alzheimers Dis 2012
- Lindberg et al. AJNR Am J Neuroradiol 2012
- Lindberg et al. Front Aging Neurosci 2012
- Walterfang et al. J Alzheimers Dis 2014
Methodology: prospective, cross sectional study, multicenter study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
* Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Appendix
Appendix
Fig. 5
Table 6
Table 7
Table 8
Table 9
Rights and permissions
About this article
Cite this article
Ferreira, D., Cavallin, L., Granberg, T. et al. Quantitative validation of a visual rating scale for frontal atrophy: associations with clinical status, APOE e4, CSF biomarkers and cognition. Eur Radiol 26, 2597–2610 (2016). https://doi.org/10.1007/s00330-015-4101-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4101-9