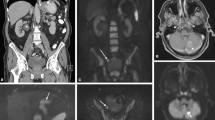
Abstract
Objectives
To evaluate whole-body MRI with diffusion-weighted sequence (WB-DWI/MRI) for staging and assessing operability compared with CT and FDG-PET/CT in patients with suspected ovarian cancer.
Methods
Thirty-two patients underwent 3-T WB-DWI/MRI, 18 F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) and CT before diagnostic open laparoscopy (DOL). Imaging findings for tumour characterisation, peritoneal and retroperitoneal staging were correlated with histopathology after DOL and/or open surgery. For distant metastases, FDG-PET/CT or image-guided biopsies were the reference standards. For tumour characterisation and peritoneal staging, WB-DWI/MRI was compared with CT and FDG-PET/CT. Interobserver agreement for WB-DWI/MRI was determined.
Results
WB-DWI/MRI showed 94 % accuracy for primary tumour characterisation compared with 88 % for CT and 94 % for FDG-PET/CT. WB-DWI/MRI showed higher accuracy of 91 % for peritoneal staging compared with CT (75 %) and FDG-PET/CT (71 %). WB-DWI/MRI and FDG-PET/CT showed higher accuracy of 87 % for detecting retroperitoneal lymphadenopathies compared with CT (71 %). WB-DWI/MRI showed excellent correlation with FDG-PET/CT (κ = 1.00) for detecting distant metastases compared with CT (κ = 0.34). Interobserver agreement was moderate to almost perfect (κ = 0.58–0.91).
Conclusions
WB-DWI/MRI shows high accuracy for characterising primary tumours, peritoneal and distant staging compared with CT and FDG-PET/CT and may be valuable for assessing operability in ovarian cancer patients.
Key Points
• Whole-body MRI with diffusion weighting (WB-DWI/MRI) helps to assess the operability of suspected ovarian cancer.
• Interobserver agreement is good for primary tumour characterisation, peritoneal and distant staging.
• WB-DWI/MRI improves mesenteric/serosal metastatic spread assessment compared with CT and FDG-PET/CT.
• Retroperitoneal/cervical-thoracic nodal staging using qualitative DWI criteria was reasonably accurate.
• WB-DWI/MRI and FDG-PET/CT showed the highest diagnostic impact for detecting thoracic metastases.






Similar content being viewed by others
Abbreviations
- WB-DWI/MRI:
-
whole-body MRI with diffusion-weighted sequence
- DOL:
-
diagnostic open laparoscopy
- PC:
-
peritoneal carcinomatosis
- FIGO:
-
International Federation of Gynaecology and Obstetrics
- NACT:
-
neoadjuvant chemotherapy
- CA:
-
cancer antigen
- MPR:
-
multiplanar reformatting
- TSE:
-
turbo spin-echo
- SI:
-
signal intensity
- PPV:
-
positive predictive value
- NPV:
-
negative predictive value
References
Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46:765–781
Vergote I, Tropé CG, Amant F et al (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943–953
Vergote I, De Wever I, Tjalma W, Van Gramberen M, Decloedt J, Van Dam P (1998) Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol 71:431–436
Zivanovic O, Eisenhauer EL, Zhou Q et al (2008) The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol 108:287–292
Aletti GD, Dowdy SC, Podratz KC, Cliby WA (2006) Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol 100:283–287
Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ (2002) Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20:1248–1259
Chi DS, Eisenhauer EL, Lang J et al (2006) What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 103:559–564
Du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzin. Cancer 115:1234–1244
Eisenkop SM, Friedman RL, Wang HJ (1998) Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol 69:103–108
Forstner R, Sala E, Kinkel K, Spencer JA (2010) ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol 20:2773–2780
Kumar D, Kand P, Basu S (2012) Impact of FDG-PET and -PET/CT imaging in the clinical decision-making of ovarian carcinoma: an evidence-based appraoch. Womens Health 8:191–203
Kyriazi S, Kaye SB, deSouza NM (2010) Imaging ovarian cancer and peritoneal metastases–current and emerging techniques. Nat Rev Clin Oncol 7:381–393
Vergote I, Van Gorp T, Amant F, Leunen K, Neven P, Berteloot P (2008) Timing of debulking surgery in advanced ovarian cancer. Int J Gynecol Cancer 18(Suppl 1):11–19
Coakley FV, Choi PH, Gougoutas CA et al (2002) Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology 223:495–499
Pannu HK, Bristow RE, Frederick J, Fishman EK (2003) Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics 23:687–701
Pannu HK, Horton KM, Fishman EK (2003) Thin section dual-phase multidetector-row computed tomography detection of peritoneal metastases in gynecologic cancers. J Comput Assist Tomogr 27:333–340
Tempany CM, Zou KH, Silverman SG, Brown DL, Kurtz AB, McNeil BJ (2000) Staging of advanced ovarian cancer: comparison of imaging modalities—report from the Radiological Diagnostic Oncology Group. Radiology 215:761–767
Low RN, Barone RM (2012) Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 19:1394–1401
Espada M, Garcia-Flores JR, Jimenez M et al (2013) Diffusion-weighted magnetic resonance imaging evaluation of intra-abdominal sites of implants to predict likelihood of suboptimal cytoreductive surgery in patients with ovarian carcinoma. Eur Radiol 23:2636–2642
Heintz APM, Odicino F, Maisonneuve P et al (2006) Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 95(Suppl 1):S161–S192
Timmerman D, Ameye L, Fischerova D et al (2010) Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 341:c6839–c6839
Vergote I, Du Bois A, Amant F, Heitz F, Leunen K, Harter P (2013) Neoadjuvant chemotherapy in advanced ovarian cancer: On what do we agree and disagree? Gynecol Oncol 128:6–11
Arrivé L, Coudray C, Azizi L et al (2007) Pineapple juice as a negative oral contrast agent in magnetic resonance cholangiopancreatography]. [Article in French]. J Radiol 88:1689–1694
Riordan RD (2004) Pineapple juice as a negative oral contrast agent in magnetic resonance cholangiopancreatography: a preliminary evaluation. Br J Radiol 77:991–999
Kolev V, Mironov S, Mironov O et al (2010) Prognostic significance of supradiaphragmatic lymphadenopathy identified on preoperative computed tomography scan in patients undergoing primary cytoreduction for advanced epithelial ovarian cancer. Int J Gynecol Cancer 20:979–984
Forstner R (2007) Radiological staging of ovarian cancer: imaging findings and contribution of CT and MRI. Eur Radiol 17:3223–3235
Kitajima K, Murakami K, Yamasaki E et al (2008) Diagnostic accuracy of integrated FDG-PET/contrast-enhanced CT in staging ovarian cancer: comparison with enhanced CT. Eur J Nucl Med Mol Imaging 35:1912–1920
Thomassin-Naggara I, Toussaint I, Perrot N et al (2011) Characterization of complex adnexal masses: value of adding perfusion- and diffusion-weighted MR imaging to conventional MR imaging. Radiology 258:793–803
Ohno Y, Koyama H, Yoshikawa T et al (2011) N stage disease in patients with non-small cell lung cancer: efficacy of quantitative and qualitative assessment with STIR turbo spin-echo imaging, diffusion-weighted MR imaging, and fluorodeoxyglucose PET/CT. Radiology 261:605–615
Kwee TC, Takahara T, Ochiai R, Nievelstein RAJ, Luijten PR (2008) Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 18:1937–1952
Padhani AR, Liu G, Mu-koh D et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Low RN, Sebrechts CP, Barone RM, Muller W (2009) Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings–a feasibility study. AJR Am J Roentgenol 193:461–470
Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH (1993) Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 72:1631–1636
De Bree E, Koops W, Kröger R, Van Ruth S, Witkamp AJ, Zoetmulder FAN (2004) Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol 86:64–73
Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu SVCM (2004) Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 22:275–282
Soussan M, Des Guetz G, Barrau V et al (2012) Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol 22:1479–1487
Turlakow A, Yeung HW, Salmon AS, Macapinlac HA, Larson SM (2003) Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med 44:1407–1412
Yoshida Y, Kurokawa T, Kawahara K et al (2004) Incremental benefits of FDG positron emission tomography over CT alone for the preoperative staging of ovarian cancer. AJR Am J Roentgenol 182:227–233
Dirisamer A, Schima W, Heinisch M et al (2009) Detection of histologically proven peritoneal carcinomatosis with fused 18 F-FDG-PET/MDCT. Eur J Radiol 69:536–541
Kim HW, Won KS, Zeon SK, Ahn BC, Gayed IW (2013) Peritoneal carcinomatosis in patients with ovarian cancer: enhanced CT versus 18 F-FDG PET/CT. Clin Nucl Med 38:93–97
Pfannenberg AC, Aschoff P, Brechtel K et al (2007) Value of contrast-enhanced multiphase CT in combined PET/CT protocols for oncological imaging. Br J Radiol 80:437–445
Satoh Y, Ichikawa T, Motosugi U et al (2011) Diagnosis of peritoneal dissemination: comparison of 18 F-FDG PET/CT, diffusion-weighted MRI, and contrast-enhanced MDCT. AJR Am J Roentgenol 196:447–453
Nakai G, Matsuki M, Inada Y et al (2008) Detection and evaluation of pelvic lymph nodes in patients with gynecologic malignancies using body diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr 32:764–768
Klerkx WM, Heintz APM, Mali WPT et al (2009) Lymph node detection by MRI before and after a systematic pelvic lymphadenectomy. Gynecol Oncol 114:315–318
Klerkx WM, Mali WM, Heintz AP, De Kort GA, Takahara T, Peeters PH (2011) Observer variation of magnetic resonance imaging and diffusion weighted imaging in pelvic lymph node detection. Eur J Radiol 78:71–74
Kwee TC, Takahara T, Luijten PR, Nievelstein RAJ (2010) ADC measurements of lymph nodes: inter- and intra-observer reproducibility study and an overview of the literature. Eur J Radiol 75:215–220
Ohno Y, Hatabu H, Takenaka D et al (2004) Metastases in mediastinal and hilar lymph nodes in patients with non-small cell lung cancer: quantitative and qualitative assessment with STIR turbo spin-echo MR imaging. Radiology 231:872–879
Low RN (2009) Diffusion-weighted MR imaging for whole body metastatic disease and lymphadenopathy. Magn Reson Imaging Clin N Am 17:245–261
Hynninen J, Auranen A, Carpén O et al (2012) FDG PET/CT in staging of advanced epithelial ovarian cancer: frequency of supradiaphragmatic lymph node metastasis challenges the traditional pattern of disease spread. Gynecol Oncol 126:64–68
Acknowledgments
This research was supported by the Belgian Foundation against Cancer (Stichting Tegen Kanker – Fondation Contre le Cancer).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
Comparative per-site accuracy for WB-DWI/MRI, CT and FDG-PET/CT (DOCX 36.8 kb)
Rights and permissions
About this article
Cite this article
Michielsen, K., Vergote, I., Op de beeck, K. et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: a clinical feasibility study in comparison to CT and FDG-PET/CT. Eur Radiol 24, 889–901 (2014). https://doi.org/10.1007/s00330-013-3083-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-3083-8