Abstract
Objective
To quantify radiation exposure and mortality risk from computed tomography (CT) and positron emission tomography (PET) imaging with 18F-fluorodeoxyglucose (18F-FDG) in patients with malignant lymphoma (Hodgkin’s disease [HD] or non-Hodgkin’s lymphoma [NHL]).
Methods
First, organ doses were assessed for a typical diagnostic work-up in children with HD and adults with NHL. Subsequently, life tables were constructed for assessment of radiation risks, also taking into account the disease-related mortality.
Results
In children with HD, cumulative effective dose from medical imaging ranged from 66 mSv (newborn) to 113 mSv (15 years old). In adults with NHL the cumulative effective dose from medical imaging was 97 mSv. Average fractions of radiation-induced deaths for children with HD [without correction for disease-related mortality in brackets] were 0.4% [0.6%] for boys and 0.7% [1.1%] for girls, and for adults with NHL 0.07% [0.28%] for men and 0.09% [0.37%] for women.
Conclusion
Taking into account the disease-related reduction in life expectancy of patients with malignant lymphoma results in a higher overall mortality but substantial lower incidence of radiation induced deaths. The modest radiation risk that results from imaging with CT and 18F-FDG PET can be considered as justified, but imaging should be performed with care, especially in children.
Key Points
-
Survival of malignant lymphoma has improved dramatically over the past decades.
-
PET and CT currently play important roles for malignant lymphoma patients.
-
The potential hazard of ionising radiation has become an increasingly important issue.
-
When assessing radiation risks, disease-related reduction in life expectancy should be considered.
-
CT and 18 F-FDG PET create a modest radiation-induced mortality risk.
Similar content being viewed by others
Introduction
Imaging plays an important role in the management of patients with malignant lymphoma (Hodgkin’s disease [HD] and non-Hodgkin’s lymphoma [NHL]). Accurate imaging is important for determining the stage of disease, which guides the treatment strategy and influences the prognosis [1]. Imaging is also important for assessing response to therapy and detecting tumour persistence or recurrence [2–4]. Computed tomography (CT) and, more recently, positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) have become indispensable tools in oncological imaging [2, 5–9]. However, CT and 18F-FDG PET result in exposure of the patient to ionising radiation, which is associated with a carcinogenic risk [10–17].
There is a worldwide growing concern about radiation exposure in medical imaging [12, 18–21]. Estimates of the National Council on Radiation Protection and Measurements (NCRP) Scientific Committee in the United States in 2006 show an almost sixfold increase in the per capita dose from medical exposure to about 3 mSv compared with 1982. CT and nuclear medicine examinations are the largest contributors [22].
Therapeutic advances have dramatically improved the survival of patients with malignant lymphoma over the past decades. HD can nowadays be cured in at least 80% of patients [23–25]. Therefore, current treatment strategies not only aim at maximising curative success but also at minimising (late) toxicity, such as infertility, premature menopause, cardiac disease, and most importantly, risk of second neoplasms [24, 25]. In this context, the potential hazard of ionising radiation that is associated with diagnostic CT and PET in patients with malignant lymphoma is an increasingly important issue. Therefore, performing dose and risk assessment should be prioritised for circumstances with high cumulative radiation exposure for individual patients and better survival rates. This is particularly true when patients are young, such as children with HD, and when the cumulative radiation dose from imaging is expected to fall within the range of 5–150 mSv, such as during follow-up of patients treated for malignant lymphoma [10–13, 15]. This latter dose range is based on the studies in the subgroup of atomic bomb survivors who received low doses of radiation, ranging from 5 to 150 mSv (mean dose of 40 mSv), which suggested a significant increase in the overall risk of cancer in this subgroup [14–16].
The aim of this study was to perform accurate radiation risk assessment for imaging of patients with malignant lymphoma. To achieve our goal, the radiation exposure of CT and 18F-FDG PET was calculated for a typical work-up for children with HD in different age categories, and for adults with NHL. We expected that disease-related mortality would have a significant effect on the assessment of radiation risk in patients with malignant lymphoma, and that disease-related mortality would be much higher compared with radiation-induced mortality. This expectation is in accordance with a very recent publication by Brenner et al. [26]. Advanced radiation risk assessment, based on the demographic methodology of life tables, was developed to take into account these effects.
Materials and methods
Clinical practice of imaging
Malignant lymphoma comprises a heterogeneous group of diseases, differing with regard to histology, treatment and outcome. It is beyond the scope of this study to encompass all the different entities. Instead we focus on the most common types of malignant lymphoma; i.e. HD in children and diffuse large B-cell lymphoma (DLBCL) in adults [27–30]. The most typical imaging strategy for children with HD (age <18 years) and for adults with DLBCL was used in this study. In children with HD, the imaging strategy was based on the protocol according to the Children’s Oncology Group [COG]; in adults with DLBCL, the imaging strategy was based on the HOVON 84 international multicentre trial currently running in The Netherlands [www.hovon.nl, EudraCTnr. 2006-005174-42] (Table 1).
Imaging with ultrasound and chest radiography were not considered in this study, as ultrasound is not associated with radiation exposure, and the radiation exposure from chest radiography is negligible compared with CT and 18F-FDG PET.
Radiation exposure from CT
There was no information available that allowed for assessment of organ doses in paediatric patients undergoing CT. Therefore we created Medical Internal Radiation Dose (MIRD) paediatric patient models in five general categories according to age and weight: newborn (3.6 kg), 1 year old (9.7 kg), 5 years old (19.8 kg), 10 years old (33.2 kg) and 15 years old (56.8 kg). The adult MIRD patient model represents an average-sized patient of 74 kg. All these hermaphrodite patient models (MIRD V) are described by spheres, ellipsoids and cones, as illustrated in Fig. 1 [31]. For assessment of radiation exposure from CT, we used these patient models in combination with an algorithm for Monte Carlo dose calculations (ImpactMC software, version 1.0, VAMP, Erlangen, Germany) [31, 32]. The Monte Carlo dose calculations are based on a virtual model of the CT system with respect to geometry, X-ray spectrum, filtration and CT parameters. The simulation is performed on 3D voxelised versions of the MIRD V phantoms. To each voxel in the volume during the simulation, a density value and a mass attenuation coefficient are assigned corresponding to five different materials: air, lung, soft tissue, fat and bone. During the simulation of a CT examination, the energy deposited in each voxel (absorbed dose) is accumulated and saved in an additional volume. The manufacturer of the Aquilion CT system (Toshiba Medical Systems) disclosed two measured X-ray spectra (100 kV and 120 kV) and information about the design of the CT system. This information was implemented in the Monte Carlo simulation. A MatLab script (The MathWorks, Natick, MA, USA) was used to extract organ doses from the calculated dose distributions within the MIRD mathematical phantoms.
The mathematical hermaphrodite Medical Internal Radiation Dose (MIRD) patient models; their body, skeleton and organs are described by spheres, ellipsoids and cones. The paediatric patient models are divided into five categories according to their age and weight: newborn (3.6 kg, left first row); 1 year old (9.7 kg); 5 years old (19.8 kg); 10 years old (33.2 kg); 15 years old (56.8 kg). The adult model represents an average-sized patient of 74 kg (right second row)
In CT it is common practice to adjust acquisition parameters to the size of the patient, and to the clinical application [33]. The acquisition protocol we used was based on local and optimised practices, and was checked against general recommendations, particularly with regard to the optimisation of the paediatric acquisitions. We thus derived the following clinical CT acquisition parameters. For small children, a tube voltage of 100 kV was used (weight <30 kg), and for larger children and adults a tube voltage of 120 kV (≥ 30 kg), both in combination with a pitch factor of 0.83. Radiation output of the CT system is expressed as the volume computed tomography dose index (CTDIvol), but also the tube charge (mAs) is provided for scans that are performed with an Aquilion 64 CT system (Toshiba Medical Systems, Japan) [34]. The clinically applied tube current, rotation time and pitch factor are associated with CTDIvols of 2.1 mGy (newborn, 3.6 kg, 27 mAs), 2.9 mGy (1 year old, 9.7 kg, 38 mAs), 4.1 mGy (5 years old, 19.8 kg, 53 mAs), 4.6 mGy (10 years old, 33.2 kg. 37 mAs), 7.1 mGy (15 years old, 56.8 kg, 58 mAs), and 9.5 mGy (adult, 74 kg, 77 mAs).
Dose calculations that were performed for the MIRD phantoms with the Monte Carlo software yielded organ doses, total body dose and effective dose according to ICRP 103. Dose assessment was performed for 12 CT acquisitions, i.e. both for whole-body CT (including neck, chest and abdomen), and for CT of the neck and chest only, in all six age categories. These doses were incorporated into the risk model, as described under “Risk assessment”. From these results, appropriate organ doses and the effective dose were derived for the year of diagnosis and treatment and for the following years of surveillance. If necessary, linear interpolation of dose values was performed to yield dose estimations at ages not included in the table.
Radiation exposure from 18F-FDG PET
For assessment of organ dose and effective dose from 18F-FDG PET, published tables were used that provide information about organ dose and effective dose per MBq of administered 18F-FDG activity. The ICRP provides information for 1-, 5-, 10- and 15-year-old children, and for adults [35]. Ruotsalainen et al. [36] published tables for estimation of radiation dose to the newborn in 18F-FDG PET studies. For PET a dose of 3 MBq of 18F-FDG per kg body weight was assumed to be administered, based on the current state-of art imaging with integrated PET/CT systems (f.i. Biograph 40 TruePoint PET-CT, Siemens Medical Systems, Knoxville, TN, USA) . The five different age categories in the children being analysed in this study correspond to 18F-FDG doses of 10 MBq (newborn), 30 MBq (1 year old), 60 MBq (5 years old), 100 MBq (10 years old) and 170 MBq (15 years old). In adults, administration of 220 MBq 18F-FDG was assumed.
Risk assessment
We performed risk assessment for five age categories of male and female paediatric patients diagnosed with HD (newborns, and children at the age of 1, 5, 10 and 15 years) and in three age categories for adult male and female patients diagnosed with DLBCL at the ages of 55, 65 and 75 years.
The BEIR VII excess relative risk (ERR) model was used for calculating radiation risk [37]. Chapter 12 of this BEIR report provides the equations and parameters for estimating organ-specific solid cancer mortality and leukaemia mortality. The ERR is expressed as a function of gender, absorbed organ dose, age at exposure and the attained age, and includes three organ-specific fit parameters. The organs are stomach, colon, liver, lung, breast, prostate, uterus, ovary, bladder, other organs and thyroid.
In the BEIR VII ERR model, the organ-specific excess risk for solid cancer mortality is expressed, relative to the gender- and age-dependent risk of the background cancer mortality for specific organs. This background is the naturally occurring mortality, not the mortality that is induced by radiation exposure during medical imaging. Data on the risk of naturally occurring cancer mortality depending on organ, gender and attained age were derived from ICRP Publication 103 (Euro-American cancer mortality rates by age and site) [38]. Subsequently, according to the BEIR VII model, an overall ERR function depending on organ dose, gender, age at exposure and attained age was calculated for male and female patients. Organ dose was incorporated into the risk model as a function of attained age; this was required because patients have different weights (and ages) and thus receive different associated radiation exposures during diagnosis, treatment and surveillance of malignant lymphoma. In addition to radiation risk, mortality rates that are typical for the young patient group with HD and the adult patient group with DLBCL could be taken into account, i.e. the overall 10-year survival for HD (94%) and 5-year survival for DLBCL (58%) [27–30].
Risk calculations were performed using life tables, and they were done with and without taking into account the disease-related mortality. Life tables (also called mortality tables) are used in demography for measuring and modelling population processes. A life table shows, for each age, what the probability is that a person of that age will die before his or her next birthday. They allow for the calculation of the fraction of radiation-induced deaths, the reduction of life expectancy and the survival rate. An essential component of the life table is age- and gender-dependent mortality; in this study three different sources of mortality were integrated in the life tables: the background, the radiation induced, and the disease-related mortality. The background mortality that is typical for the asymptomatic population (gender- and age-specific probability of dying) was derived for the European population at large based on the Eurostat database [39]. The radiation-induced, age-, gender- and dose-dependent overall mortality that was calculated with the BEIR VII model as described in the previous section, was also incorporated into the life tables. Finally, disease-related mortality, as mentioned in the previous section, can also be integrated in the life tables. Calculations were carried out with the life tables with and without taking into account the mortality risks that are typical for patients with malignant lymphoma (HD- and DLBCL-related mortality rates).
Results
Radiation exposure from CT and 18F-FDG PET
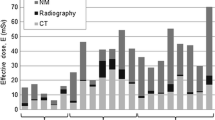
The radiation exposure from whole-body CT, from CT of the neck and thorax, and from whole-body 18F-FDG PET for children (HD) according to the five age categories and for adults (DLBCL) are presented in Table 2. Effective dose increased gradually with age from 5.5 mSv to 13.3 mSv (whole-body CT), and from 3.4 mSv to 7.9 mSv (neck-chest CT). For 18F-FDG PET examinations the variations in effective dose were smaller, ranging from 2.8 mSv to 4.3 mSv. In CT the highest organ doses were observed for lung and thyroid, and variations in organ dose were modest for organs lying entirely within the anatomical area examined. In PET the highest organ dose by far was observed for the bladder.
Neonates and children with HD usually undergo seven whole-body CTs, five of the neck and chest, and two 18F-FDG PETs over a period of 5 years. Adults with DLBCL usually undergo seven whole-body CTs and one 18F-FDG PET during a period of 2.5 years. Because of the intensive use of X-ray imaging, the effective dose accumulates rapidly in patients with malignant lymphoma. In children with HD, cumulative effective dose reaches 66 mSv (newborn at diagnosis), 69 mSv (1 year old at diagnosis), 80 mSv (5 years old at diagnosis), 90 mSv (10 years old at diagnosis) and 113 mSv (15 years old at diagnosis). Differences between these paediatric age categories are due to differences in patient size and the patient size-specific acquisition protocols for both CT and 18F-FDG PET. In adults with DLBCL the cumulative effective dose reaches 97 mSv 2.5 years after diagnosis.
Risk assessment
Demographic and risk-related parameters were derived from the life tables for neonates and children (with HD) corresponding to the five different age and weight categories. The paediatric categories are identified by the age at diagnosis (Table 3) and for adult patients (with DLBCL) the categories were 55, 65 and 75 years of age at diagnosis of the disease (Table 4).
Calculations were performed with and without taking into account the disease-related mortality rates typical for the patient cohorts (Table 3). Risk assessment taking into account the effects of disease-related mortality resulted in a substantially lower fraction of radiation-induced deaths, and a lower associated reduction of life expectancy.
The calculations, which included disease-related mortality [or without disease-related mortality in brackets], resulted in an average 10-year survival of 94% (range 93–94%) [100% (range 99-100%)] for the young patient group with HD and an average 5-year survival of 55% (range 46–59%) [90% (range 76-98%)] for the adult patient group with DLBCL. Within the paediatric patient group, the average fraction of radiation-induced deaths was 0.4% [0.6%] for boys and 0.7% [1.1%] for girls. Within the adult group these values were even smaller; 0.07% [0.28%] for men and 0.09% [0.37%] for women. The average radiation-induced reduction of life expectancy in paediatric patients with HD is 21 days for boys and 45 days for girls. In adults with DLBCL, the average radiation-induced reduction of life expectancy is 1.5 days in men and 2.0 days in women.
Discussion
This study shows that the effect of disease-related mortality on radiation risk assessment in patients with malignant lymphoma is substantial. By taking into account disease-related mortality, the proportion of radiation-induced deaths decreased between 30% and 40% in paediatric patients with HD, and between 50% and 80% in adults with DLBCL. Radiation-induced reduction of life expectancy decreased by similar percentages. Our methodology, which integrates a model for organ dose assessment and risk assessment in one demographic model, showed that the potential radiation-induced reduction of life expectancy is only a small fraction compared with the disease-related reduction of life expectancy, namely a fraction between 0.003 and 0.011 for HD (children); and between 0.0003 and 0.0006 for DLBCL (adults). This reflects the fact that the radiation-related risk is a late risk with its expression up to decades after the actual exposure and has therefore less effect on the reduction of life expectancy. Our methodology for radiation risk assessment is an improvement compared with the usual standard of practice, where disease-related mortality is not considered. Although the observed cumulative effective doses are relatively high for imaging patients with malignant lymphoma, the associated estimated radiation risks are still very modest.
Within the paediatric patient group, it is estimated that, on average, 0.4% of male and 0.7% of female patients eventually die because of the radiation exposure associated with medical imaging. For adult patients diagnosed with DLBCL, it is estimated that 0.07% of male and 0.09% of female patients die because of radiation exposure. The higher values for women result mainly from the relatively high sensitivity of the female breast to radiation exposure [40].
Calculation in which the disease-related mortality was taken into account resulted in an average 10-year survival of 94% (range, 93–94%) for the young patient group with HD, which matches excellently with the published overall 10-year survival of 94% [27, 28]. Similarly, for the adult patient group with DLBCL, the calculated average 5-year survival was 55% (range, 46–59 %), which matched well with the published value of 58% [29, 30]. The good agreement between our calculated reduction in survival after diagnosis and published values is a clear indication that risk assessment should be performed taking disease-related mortality into account.
This study has some limitations. Malignant lymphoma comprises a heterogeneous group of entities, with more than 40 subtypes [41]. In addition, with regard to radiation exposure, age is an important factor. Therefore we chose to analyse the risk of radiation exposure in only two types of lymphoma, HD in paediatric patients and DLBCL in adult patients (the most common malignant lymphoma subtypes in these age groups). Another limitation is that the assumed 10- and 5-year survival rates (for HD and DLBCL, respectively) may be an underestimation of the current situation, because treatment strategies have continued to evolve [27–30]. In this study, we focused on the mortality risk caused by the radiation exposure of the patient. The morbidity risk induced by the radiation exposure was not considered. It can be assumed that not all patients with a radiation-induced malignancy die from this malignancy, and more patients suffer from radiation-induced diseases than the number of patients expressed in the mortality risk. Our calculations are based on a methodology that is according to broadly accepted dosimetric techniques and risk models. However, radiation dose assessment and risk assessment at the low dose levels that are common in diagnostic radiology are always associated with considerable uncertainties, implying that the absolute risk figures that we calculated should be interpreted with care.
Furthermore, with the implementation of integrated PET-CT systems, low-dose CT is performed in addition to PET, as it is used for attenuation correction in PET imaging. The use of CT for attenuation correction reduces the examination time, which implies a lower dose of FDG (5 MBq/kg vs 3 MBq/kg). The low-dose CT was not included in this study. Two or one additional low-dose CT(s) would have been performed in children with HD and in adults with DLBCL, respectively, with each low-dose CT causing extra radiation exposure of only approximately 3 mSv for adults [42]. Finally, it should be recognised that there are considerable uncertainties in the radiation risk model of BEIR, especially regarding the risk at low-dose levels such as those encountered in CT [17].
In order to establish the most efficient imaging strategy and limit the radiation exposure in patients with malignant lymphoma, there are different options. It may be sufficient to use only the low-dose whole-body CT (combined with FDG-PET) during and after therapy instead of a diagnostic (i.e. full-dose) whole body CT. This implies a reduction in radiation exposure of 6 times 8 mSv for adults. In patients with a FDG-avid type of malignant lymphoma and a baseline 18F-FDG PET-CT combined with a diagnostic CT, the most accurate and appropriate method of imaging during follow-up may therefore prove to be the low dose PET-CT, without the diagnostic CT. Another recent development is the introduction of whole-body magnetic resonance imaging (MRI) for the evaluation of malignant lymphoma. Initial results on this application of whole-body MRI are promising, but more research is still needed before it can be recommended as an alternative to CT and/or 18F-FDG PET [43–46].
In conclusion, the disease-related reduction in life expectancy of patients diagnosed with malignant lymphoma must be taken into account to achieve more realistic estimates of radiation risk. It results in higher overall mortality and substantial lower incidence of radiation-induced deaths. Although the cumulative effective dose from medical imaging is high, the actual calculated radiation risks are very modest. The radiation exposure that results from imaging with CT and 18F-FDG PET is considered as justified in patients with malignant lymphoma, but should still be performed with care, especially in children. Ongoing studies have to establish the most efficient imaging strategies for (the different subtypes of) malignant lymphoma.
References
Kwee TC, Kwee RM, Nievelstein RA (2008) Imaging in staging of malignant lymphoma: a systematic review. Blood 111:504–516
Hoskin PJ (2003) PET in lymphoma: what are the oncologist’s needs? Eur J Nucl Med Mol Imaging Supply 1:S37–S41
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M (1971) Report of the committee on Hodgkin’s disease staging classification. Cancer Res 11:1860–1861
Armitage JO (2005) Staging non-Hodgkin lymphoma. CA Cancer J Clin 55:368–376
Hoh CK, Glaspy J, Rosen P et al (1997) Whole-body FDG-PET imaging for staging of Hodgkin's disease and lymphoma. J Nucl Med 38:343–348
Buchmann I, Reinhardt M, Elsner K et al (2001) 2-(fluorine-18)fluoro-2-deoxy-D-glucose positron emission tomography in the detection and staging of malignant lymphoma. A bicenter trial. Cancer 91:889–899
Seam P, Juweid ME, Cheson BD (2007) The role of FDG-PET scans in patients with lymphoma. Blood 110:3507–3516
Juweid ME, Stroobants S, Hoekstra OS et al (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol 25:571–578
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176:289–296
Brenner DJ, Elliston CD (2004) Estimated radiation risks potentially associated with full-body CT screening. Radiology 232:735–738
Linton OW, Mettler FA Jr (2003) National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol 181:321–329
Executive Summary Board on Radiation Effects Research—Division on Earth and Life Studies (2005) Health risks from exposure to low levels of ionizing radiation: BEIR VII—Phase 2. National Academy Press, Washington
Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K (2003) Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 160:381–407
Pierce DA, Preston DL (2000) Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res 154:178–186
Preston DL, Ron E, Tokuoka S et al (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168:1–64
National Council on Radiation Protection and Measurements (1997) Uncertainties in fatal cancer risk estimates used in radiation protection. Report 126. NCRP, Bethesda
Amis ES Jr, Butler PF, Applegate KE et al (2007) American college of radiology white paper on radiation dose in medicine. J Am Coll Radiol 4:272–284
Hall EJ, Brenner DJ (2008) Cancer risks from diagnostic radiology. Br J Radiol 81:362–378
Hillman BJ (2008) Radiation exposure and imaging utilization. J Am Coll Radiol 5:689–690
Mezrich R (2008) Are CT scans carcinogenic? J Am Coll Radiol 5:691–693
Mettler FA Jr, Thomadsen BR, Bhargavan M et al (2008) Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys 95:502–507
Connors JM (2005) State-of-the-art therapeutics: Hodgkin’s lymphoma. J Clin Oncol 23:6400–6408
Ansell SM, Armitage J (2005) Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc 80:1087–1097
Hennessy BT, Hanrahan EO, Daly PA (2004) Non-Hodgkin lymphoma: an update. Lancet Oncol 5:341–353
Brenner DJ, Shuryak I, Einstein AJ (2011) Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology 261:193–198
von der Weid NX (2008) Adult life after surviving lymphoma in childhood. Support Care Cancer 16:339–345
Chow LM, Nathan PC, Hodgson DC et al (2006) Survival and late effects in children with Hodgkin’s lymphoma treated with MOPP/ABV and low-dose, extended-field irradiation. J Clin Oncol 24:5735–5741
Illidge T, Tolan S (2008) Current treatment approaches for diffuse large B-cell lymphoma. Leuk Lymphoma 49:663–676
Feugier P, Van HA, Sebban C et al (2005) Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 23:4117–4126
Cristy M (1980) Mathematical phantoms representing children of various ages for use in estimates of internal dose. U.S. Nuclear Regulatory Commission Rep. NUREG/CR-1159 (also Oak Ridge National Laboratory Rep. ORNL/NUREG/TM-367)
Deak P, van Straten SM, Shrimpton PC, Zankl M, Kalender WA (2008) Validation of a Monte Carlo tool for patient-specific dose simulations in multi-slice computed tomography. Eur Radiol 18:759–772
Nievelstein RA, van Dam IM, van der Molen AJ (2010) Multidetector CT in children: current concepts and dose reduction strategies. Pediatr Radiol 40:1324–1344
Nitt-Gray MF (2002) AAPM/RSNA physics tutorial for residents: topics in CT. Radiation dose in CT. Radiographics 22:1541–1553
Valentin DJ (1998) Radiation dose to patients from radiopharmaceuticals (Addendum 2 to ICRP Publication 53). Ann ICRP 28:1
Ruotsalainen U, Suhonen-Polvi H, Eronen E et al (1996) Estimated radiation dose to the newborn in FDG-PET studies. J Nucl Med 37:387–393
NAS/NRC (2006) Health risks from exposure to low levels of ionising radiation: BEIR VII phase 2. Board on Radiation Effects Research National Research Council of the National Academies, Washington
The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37:1–332
Eurostat (2007) http://epp.eurostat.ec.europa.eu/
De Bruin ML, Sparidans J, van’t Veer MB et al (2009) Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 27:4239–4246
Swerdlow S, Campo E, Harris N (2008) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. International Agency for Research on Cancer, Lyon
Brix G, Lechel U, Glatting G et al (2005) Radiation exposure of patients undergoing whole-body dual-modality 18 F-FDG PET/CT examinations. J Nucl Med 46:608–613
Kellenberger CJ, Miller SF, Khan M, Gilday DL, Weitzman S, Babyn PS (2004) Initial experience with FSE STIR whole-body MR imaging for staging lymphoma in children. Eur Radiol 14:1829–1841
Brennan DD, Gleeson T, Coate LE, Cronin C, Carney D, Eustace SJ (2005) A comparison of whole-body MRI and CT for the staging of lymphoma. AJR Am J Roentgenol 185:711–716
Kwee TC, Quarles van Ufford HM, Beek FJ et al (2009) Whole-body MRI, including diffusion-weighted imaging, for the initial staging of malignant lymphoma: comparison to computed tomography. Invest Radiol 44:683–690
Quarles van Ufford HM, Kwee TC, Beek FJ et al (2011) Newly diagnosed lymphoma: initial results with whole-body T1-weighted, STIR, and diffusion-weighted MRI compared with 18 F-FDG PET/CT. AJR Am J Roentgenol 196:662–669
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Nievelstein, R.A.J., Quarles van Ufford, H.M.E., Kwee, T.C. et al. Radiation exposure and mortality risk from CT and PET imaging of patients with malignant lymphoma. Eur Radiol 22, 1946–1954 (2012). https://doi.org/10.1007/s00330-012-2447-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2447-9