Abstract
Radioembolization with yttrium-90 microspheres (90Y-RE), either glass- or resin-based, is increasingly applied in patients with unresectable liver malignancies. Clinical results are promising but overall response and survival are not yet known. Therefore a meta-analysis on tumor response and survival in patients who underwent 90Y-RE was conducted. Based on an extensive literature search, six groups were formed. Determinants were cancer type, microsphere type, chemotherapy protocol used, and stage (deployment in first-line or as salvage therapy). For colorectal liver metastases (mCRC), in a salvage setting, response was 79% for 90Y-RE combined with 5-fluorouracil/leucovorin (5-FU/LV), and 79% when combined with 5-FU/LV/oxaliplatin or 5-FU/LV/irinotecan, and in a first-line setting 91% and 91%, respectively. For hepatocellular carcinoma (HCC), response was 89% for resin microspheres and 78% for glass microspheres. No statistical method is available to assess median survival based on data presented in the literature. In mCRC, 90Y-RE delivers high response rates, especially if used neoadjuvant to chemotherapy. In HCC, 90Y-RE with resin microspheres is significantly more effective than 90Y-RE with glass microspheres. The impact on survival will become known only when the results of phase III studies are published.
Similar content being viewed by others
Introduction
Internal radiation therapy through transarterial delivery of beta-emitting yttrium-90 (90Y)-loaded microspheres, often referred to as 90Y radioembolization (90Y-RE), is an emerging technique for the treatment of patients with unresectable primary or metastatic liver tumors [1, 2]. The efficacy of this radioembolization technique is based on the fact that intrahepatic malignancies derive their blood supply almost entirely from the hepatic artery, as opposed to the normal liver, which mainly depends on the portal vein for its blood supply [3]. The microspheres are injected selectively into the proper hepatic artery and subsequently become lodged in the microvasculature surrounding the tumor. Very high irradiation doses are delivered to the tumors, whereas the surrounding liver parenchyma is largely spared [4].
Two FDA-approved 90Y microsphere products are in clinical use at present: TheraSphere® (MDS Nordion Inc., Kanata, Ontario, Canada), which are glass microspheres, and the resin-based SIR-Spheres® (SIRTeX Medical Ltd., Sydney, New South Wales, Australia) (Table 1). The glass microspheres are approved for use in radiation treatment or as a neoadjuvant to surgery or transplantation in patients with hepatocellular carcinoma (HCC). The resin microspheres have FDA premarket approval for the treatment of hepatic metastatic colorectal cancer (mCRC), with adjuvant hepatic arterial infusion of floxuridine. However, patients suffering from other liver dominant cancers have also undergone therapy with these 90Y microspheres. These include, among others, liver metastases of breast cancer, pancreatic cancer, and neuroendocrine tumors [5, 6]. Since in most studies that have been published the majority of patients underwent 90Y-RE in a salvage setting, and most of the literature comprised phase I and II studies with small patient numbers, the overall response and real impact on survival are not known. In order to assess the effect of 90Y-RE for primary and secondary liver malignancies, a systematic meta-analysis has been performed of the available literature.
Methods
Identification of studies
A comprehensive search was carried out using several databases in order to identify relevant studies from 1986 onwards. The following search strategy was used to search the MEDLINE database with PubMed: (“yttrium” [MeSH Terms] OR yttrium [Text Word]) AND (“liver” [MeSH Terms] OR liver [Text Word]). The limit “humans” was used. The EMBASE database was searched with the limit human using: (“yttrium”/exp OR “yttrium”) AND (“liver”/exp OR “liver”). The Cochrane library database was searched with the keywords “yttrium” and “liver”. The search was completed by searching the reference lists and related articles of all relevant articles found. In addition, the reference lists of two presentations given at a workshop held in Chicago 4–5 May 2007 [7] and the list of publications in the clinicians’ section of the webpage of SIRTeX Medical Ltd. [8] and the Resource Library on the webpage of MDS Nordion Inc. [9] were screened.
Inclusion and exclusion criteria
All abstracts of relevant studies were reviewed with a set of predefined inclusion and exclusion criteria. All articles from 1986 onwards which presented data concerning tumor response or survival of patients with primary or secondary liver malignancies after treatment with 90Y glass or 90Y resin microspheres were included for further data extraction. This resulted in 44 articles (Fig. 1). Articles written in a language other than English or German were excluded; articles that presented data that were thought to have been presented previously were used once. Consequently, one article was excluded because it was written in Chinese, and another was excluded, since it was thought to present data that were also presented in another larger trial. This resulted in 42 articles from which data were extracted.
Data extraction
After the initial assessment for inclusion the following data were extracted from the 42 articles selected: study design, number, and demographic data of patients; minor extrahepatic disease included/excluded, previous therapies targeted on the liver tumor, administered dosage, site of microsphere delivery, use of angiotensin II, number of microsphere treatments, (neo)adjuvant therapies, tumor response measured by CT, MRI, and/or 18F-FDG-PET, serum markers measurements (CEA, AFP), time to progression, and survival.
After initial data extraction, the exclusion criteria were reassessed. It became clear that most studies presented adequate data on patients with HCC or with mCRC, and that response was usually measured by CT. The meta-analysis was therefore limited to these two tumor types. In order to perform a meta-analysis, additional exclusion criteria were incorporated. Articles that did not present data about HCC and/or mCRC and articles only presenting data on groups with mixed primary disease were excluded from the meta-analysis. Articles that did not present tumor response measured by CT scans or that did not present data on median survival times were also excluded. Following the additional exclusion criteria, an additional 12 articles were excluded from the meta-analysis.
Data structuring
The 30 remaining articles were divided into two groups, according to tumor type, i.e., mCRC or HCC. The pathology of these two types of liver tumors is very different. Colorectal carcinoma initially metastasizes to one or a few focal parts of the liver, whereas HCC usually spreads diffusely throughout the liver. Response to chemotherapy is also very different in these tumor types. This resulted in the formation of two groups (mCRC and HCC), for which the studies were compared on design and patient population, in order to assess the comparability of the results.
In the group of patients with mCRC, after data extraction the use of different (generations of) chemotherapy regimens was identified as a major source of heterogeneity. Two covariates were therefore included in the meta-regression model: (1) whether the older generation of cytostatic agents (5-FU/LV or floxuridine) or the newer generation (5-FU/LV + oxaliplatin (FOLFOX) or 5-FU/LV + irinotecan (FOLFIRI)) was used, and (2) whether 90Y-RE was given as a salvage therapy or as a first-line treatment with adjuvant chemotherapy. No separation was made between the microsphere product that was used (glass or resin), because of the small number of patients with mCRC treated with the glass microspheres (ca. 8%).
In view of the chemoresistant nature of HCC [10], previously given therapy was not observed as a source of heterogeneity. Therefore, the main source of heterogeneity observed in this group was the microsphere product used, either glass or resin. This resulted in the formation of two subgroups.
To allow comparability of results with regard to tumor response, the category of ‘any response’ (AR) was introduced. The AR category comprises all patients originally from the categories complete response, partial response, and stable disease.
Meta-analysis
The study of Andrews et al. [11] included just one HCC patient. This patient was therefore not included in the analysis. The proportions of patients with AR were modeled by a meta-regression analysis according to Hamza et al. [12]. This method uses the exact binomial likelihood approach instead of an approximate method based on the normal distribution of within-study variability. A random effects model was applied since considerable heterogeneity was observed between the studies. The meta-regression analysis was performed using PROC NLMIXED in SAS version 9.1 as described by Hamza et al.
Results
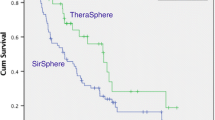
Thirty articles were included in the meta-analysis. In 999 out of 1,217 patients, tumor response was assessed by CT. The proportion of AR for HCC and mCRC combined varied between 0.29 and 1.00 with a median value of 0.82. Treatment with glass microspheres showed a lower response (AR = 0.77) than treatment with resin microspheres (AR = 0.85) (p = 0.07), with an estimated odds ratio of 0.56 (95% CI 0.29–1.06).
Colorectal liver metastases
In a total of 19 eligible studies 792 patients with mCRC had undergone 90Y-RE [6, 11, 13–29]. In 18 studies tumor response was assessed in a total of 681 patients. Of these patients 486/681 had received 90Y-RE in a salvage setting, of which 124/486 had been previously treated and/or co-treated with 5-FU/LV or floxuridine, and 362/486 had been given the newer-generation cytostatic agents. One hundred and ninety-five patients had received 90Y-RE as a first-line treatment, of which 175/195 were treated with adjuvant 5-FU/LV or floxuridine and 20/195 with FOLFOX.
The specific cytostatic agent(s) (“old” versus “new”) that were used did not affect response (p = 0.96). Whether 90Y-RE was offered in a salvage setting or as a first-line therapy affected tumor response significantly (p = 0.07). The estimated proportions of AR, based on the regression model, were 0.79 and 0.79 in salvage setting and 0.91 and 0.91 in the first-line, for the older and newer chemotherapy, respectively.
Median survival after 90Y-RE, irrespective of differences in determinants (microspheres type, chemotherapy protocol, and stage: salvage or first-line), varied from 6.7 to 17.0 months. The reported median survival from diagnosis of mCRC ranged from 10.8 to 29.4 months (Table 2).
Two randomized controlled trials were performed in patients with unresectable mCRC. In 2001, Gray et al. presented the results for 76 patients who had been randomized to either 90Y-RE (resin) as neoadjuvant to hepatic arterial infusion (HAI) of floxuridine or to HAI alone [14]. Patients in the combination arm showed a significantly greater response when measured by tumor volume, and a significantly increased time to progression. AR was 78% and 59% (p = 0.03) for the combination arm and the HAI-alone arm, respectively, and time to progression, based on tumor area measurements, was 15.9 months vs. 9.7 months (p = 0.001), respectively. In 2004, Van Hazel et al. reported on the outcome in 21 previously untreated patients with mCRC [15] in a similar study, in which it was demonstrated that the addition of a single administration of resin microspheres prior to 5-FU/LV significantly increased response, time to progression, and survival. In this phase II trial AR was 100% in the combination arm vs. 60% in the chemotherapy-alone arm (p < 0.001), time to progression 18.6 and 3.6 months (p < 0.0005), respectively, and survival 29.4 and 12.8 months (p = 0.02). Thirty-six months postrandomization 36% of patients in the combination arm were still alive, whereas no patients from the 5-FU/LV-alone arm were alive at that time.
Hepatocellular carcinoma
In 14 articles clinical data were presented on tumor response and survival for 425 patients with HCC who had received 90Y-RE [11, 18, 24, 30–40]. Twelve studies presented data of tumor response for a total of 318 patients. Treatment with resin microspheres was associated with a significantly higher proportion of AR than glass microsphere treatment (0.89 vs. 0.78 (p = 0.02)).
Median survival was reported in seven studies in which survival time was defined as survival from treatment or from diagnosis or recurrence. Median survival from microsphere treatment varied between 7.1 and 21.0 months, and median survival from diagnosis or recurrence was 9.4–24.0 months (Table 3).
Discussion
This meta-analysis showed that in patients with mCRC the tumor response of 90Y-RE is high, with AR rates of approximately 80% in a salvage setting, and over 90% when used as first-line treatment, as neoadjuvant to chemotherapy. The response rates reported for studies in which 5FU/LV was combined with irinotecan or oxaliplatin were similar to those of studies in which only 5FU/LV was used. This can probably be explained by differences in the criteria for tumor response that were used (WHO versus RECIST criteria [41]).
Regarding the question as to which microsphere is most effective in the treatment of mCRC—glass or resin—no conclusions can be reached since only 8% of the patients with mCRC were treated with the glass microspheres. Furthermore, the meta-analysis showed that resin microspheres were significantly more effective in treating HCC than glass microspheres (AR 89% vs. 78% (p = 0.02)). This is a rather unexpected finding, because only the glass microspheres are FDA-approved for treating HCC, whereas the resin microspheres are approved for mCRC, not HCC. It may be postulated that this outcome is the consequence of the substantial difference in numbers of microspheres that are infused: a dose of glass microspheres consists of 4 million microspheres, whereas a dose of resin microspheres usually contains 50 million microspheres [42]. It has been reported in the literature that administration of resin microspheres had to be prematurely halted, before the predetermined amount of radioactivity was instilled, due to macroscopic embolization [43]. In contrast, the relatively very low number of glass microspheres per dose is associated with microscopic embolization [39]. However, the low number of particles infused in the case of the glass microspheres may be a disadvantage when targeting a tumor type that is often diffusely spread throughout the liver at the time of diagnosis [44]; the radiation dose would be distributed in and around the tumors too heterogeneously to be able to deliver a tumoricidal dose to the entire lesion even if the total amount of radioactivity of a dose of glass microspheres is at least 50% higher than is the case in the resin microspheres (Table 1). Another (theoretical) consideration is that the macroembolic effect of the resin microspheres is accompanied by a greater lack of oxygen resulting in ischemia and therefore enhanced efficacy. On the other hand, shortage of oxygen might also diminish the tumoricidal effect of ionizing radiation due to a lack of oxygen radicals that is produced in this environment.
However, this macroembolic effect can be associated with clinical signs, the so-called postembolization syndrome (PES), which is reported to frequently occur following resin microspheres infusion, but not often subsequent to administration of the minimally embolic glass microspheres. PES is characterized by fatigue, nausea, fever, right upper quadrant pain, and/or vomitus, all of which are transitory and can be effectively controlled by outpatient medication [21, 39, 45–47].
Serious complications have been reported when microspheres were inadvertently deposited in excessive amounts in organs other than the liver. Conditions that have been reported include gastrointestinal ulceration/bleeding, gastritis/duodenitis, cholecystitis, pancreatitis, and radiation pneumonitis [42, 45, 48–52]. Training, careful patient selection, meticulous pretreatment assessment, and coiling of relevant vasculature reduce complication rates massively [53]. Radiation-induced liver disease following 90Y-RE has been reported sporadically [15, 54]. Careful patient selection and individualized dose calculation minimize the risk of this complication. Profound and persistent lymphopenia, with rapid onset and in some cases lasting over 12 months, though without clinical consequences, has been reported in patients with HCC following 90Y-RE with glass microspheres [36, 38]. This complication has not been observed subsequent to 90Y-RE with resin microspheres (as monotherapy). The underlying mechanism is not clear but myelosuppression is not probable since leaching of radioactivity from the glass microspheres does not take place [55]. However, following 90Y-RE, in addition to the liver tumors and to some extent the liver parenchyma, a radiation dose is delivered to the blood each time it passes the liver, which might explain this adverse laboratory event.
Unfortunately, in this meta-analysis overall tumor response could only be assessed as ‘any response’, which is caused by the reality that response categories were not uniformly defined in the analyzed studies. It is expected that this problem of being able to compare tumor response will disappear in the near future, since the RECIST criteria, published in 2001 [41], are evermore applied. In accordance with the RECIST criteria, tumor response in malignant liver disease is assessed using cross-sectional anatomic imaging (CT, MRI), by measuring tumor size. However, lesion size reduction does not always occur even if treatment is effective. This is associated with different peri- and endotumoral processes that can occur post 90Y-RE, e.g., peritumoral edema and hemorrhage, and ring enhancement [56]. Therefore, actual tumor response may often be better than is reported, based on CT measurements alone. In a significant number of cases ‘stable disease’ could actually be minor, partial, or even complete response. In order to improve sensitivity in assessing tumor response, it is therefore strongly recommended that 18F-FDG-PET or functional MRI (diffusion-weighted MRI) is added to post-treatment response assessment protocols [56–59].
Only two randomized controlled trials were found in the literature, both on resin microspheres and mCRC. The results were encouraging, showing a major survival benefit for the 90Y-RE + chemo arm. However, since then larger controlled trials have commenced, in which more effective chemotherapeutics were used [60].
In this paper the emphasis was placed on 90Y-RE in patients with unresectable HCC and mCRC. Nonetheless, patients with liver metastases from primaries other than mCRC have been treated with 90Y-RE. This is particularly the case for liver metastasized breast cancer, of which response rates of over 90% are reported [61, 62]. 90Y-RE has been applied in patients with neuroendocrine liver metastases, too, albeit in small numbers [11, 63]. Reported response rates were 100%, and it would therefore be worthwhile to further explore the use of 90Y-RE for this indication.
Fortunately, 90Y-RE is not the only novel and effective treatment option offered to patients with unresectable HCC. Recently, a breakthrough has been reported in the field of biological agents. For sorafenib (Nexavar®, Bayer Healtcare AG, Leverkusen, Germany), an oral multikinase inhibitor, a statistically significant and clinically meaningful improvement in survival has been shown in HCC patients with advanced disease: 10.7 months in the sorafenib group versus 7.9 months in the placebo group (p = 0.0006) [64]. Recently, a phase I/II trial has started in which patients with unresectable HCC are treated with the resin microspheres plus sorafenib [60].
The clinical efficacy of other promising molecular agents, e.g., bevacizumab, erlotinib, is currently being investigated as well. When added to FOLFOX or XELOX (capecitabine + oxaliplatin), the angiogenesis inhibitor bevacizumab (Avastin®, Genentech Inc., South San Francisco, CA, USA) has been proven to prolong survival of patients with colorectal cancer by approximately 6 months compared with FOLFOX or XELOX alone [65, 66]. In fact, in an ongoing multicenter study, the “FAST” trial, patients with unresectable colorectal liver metastases are treated concurrently with FOLFOX or FOLFIRI, bevacizumab, and 90Y-RE (resin microspheres) [67].
In conclusion, 90Y-RE is associated with high response rates, both in a salvage and in a first-line setting. The true impact on survival will only become known after publication of several ongoing and/or to be initiated phase III studies. The results of trials in which 90Y-RE and modern chemotherapy agents are combined with novel biological agents are awaited with interest as well.
References
Salem R, Thurston K (2006) Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol 17:1571–1593
Vente M, Hobbelink M, van het Schip A et al (2007) Radionuclide liver cancer therapies: from concept to current clinical status. Anticancer Agents Med Chem 7:441–459
Bierman H, Byron R, Kelley K et al (1951) Studies on the blood supply of tumors in man. III. Vascular patterns of the liver by hepatic arteriography in vivo. J Natl Cancer Inst 12:107–131
Gulec S, Fong Y (2007) Yttrium 90 microsphere selective internal radiation treatment of hepatic colorectal metastases. Arch Surg 142:675–682
Murthy R, Kamat P, Nunez R et al (2008) Yttrium-90 microsphere radioembolotherapy of hepatic metastatic neuroendocrine carcinomas after hepatic arterial embolization. J Vasc Interv Radiol 19:145–151
Sato K, Lewandowski R, Mulcahy M et al (2008) Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres-safety, efficacy, and survival. Radiol 247:507–515
Jakobs T (2007) mCRC treament outcomes: US and EU data review. Presented at the emerging trends in radioembolisation using microspheres, clinical workshop, Chicago, IL, 4–5 May 2007
SIRTeX Medical Ltd (2008) Publications (international). Available via http://www.sirtex.com/content.cfm?sec=usa&MenuID=1120&ID=F4CC0AB7. Accessed 1 May 2008
MDS Nordion Inc (2008) Publications. Available via http://www.nordion.com/therasphere/physicians-publications-eu.asp. Accessed 1 May 2008
Carr B (2004) Hepatocellular carcinoma: current management and future trends. Gastroenterology 127:S218–S224
Andrews J, Walker S, Ackermann R et al (1994) Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med 35:1637–1644
Hamza T, Van Houwelingen H, Stijnen T (2008) The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol 61:41–51
Gray B, Anderson J, Burton M et al (1992) Regression of liver metastases following treatment with yttrium-90 microspheres. Aust N Z J Surg 62:105–110
Gray B, Van Hazel G, Hope M et al (2001) Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy. Ann Oncol 12:1711–1720
Van Hazel G, Blackwell A, Anderson J et al (2004) Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 88:78–85
Stubbs R, Cannan R, Mitchell A (2001) Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J Gastrointest Surg 5:294–302
Murthy R, Xiong H, Nunez R et al (2005) Yttrium 90 resin microspheres for the treatment of unresectable colorectal hepatic metastases after failure of multiple chemotherapy regimens: preliminary results. J Vasc Interv Radiol 16:937–945
Lim L, Gibbs P, Yip D et al (2005) Prospective study of treatment with selective internal radiation therapy spheres in patients with unresectable primary or secondary hepatic malignancies. Intern Med J 35:222–227
Lim L, Gibbs P, Yip D et al (2005) A prospective evaluation of treatment with selective internal radiation therapy (SIR-Spheres) in patients with unresectable liver metastases from colorectal cancer previously treated with 5-FU based chemotherapy. BMC Cancer 5:132–137
Mancini R, Carpanese L, Sciuto R et al (2006) A multicentric phase II clinical trial on intra-arterial hepatic radiotherapy with 90yttrium SIR-Spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: preliminary results on toxicity and response rates. In Vivo 20:711–714
Kennedy A, Coldwell D, Nutting C et al (2006) Resin (90)Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys 65:412–425
Stubbs R, O’Brien I, Correia M (2006) Selective internal radiation therapy with 90Y microspheres for colorectal liver metastases: single-centre experience with 100 patients. ANZ J Surg 76:696–703
Sharma R, Van Hazel G, Morgan B et al (2007) Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 25:1099–1106
Jakobs T, Hoffmann R, Poepperl G et al (2007) Mid-term results in otherwise treatment refractory primary or secondary liver confined tumours treated with selective internal radiation therapy (SIRT) using (90)Yttrium resin-microspheres. Eur Radiol 17:1320–1330
Anderson J, Goldberg J, Bessent R et al (1992) Glass yttrium-90 microspheres for patients with colorectal liver metastases. Radiother Oncol 25:137–139
Wong C, Salem R, Raman S et al (2002) Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging 29:815–820
Lewandowski R, Thurston K, Goin J et al (2005) 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135–150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol 16:1641–1651
Gray B, Van Hazel M, Buck M et al (2000) Treatment of colorectal liver metastases with SIR-Spheres plus chemotherapy. GI Cancer 3:249–257
Stubbs R, Cannan R, Mitchell A et al (1999) An initial experience with selective internal radiation therapy (SIRT) for non-resectable colorectal liver metastases. GI Cancer 3:135–143
Geschwind J, Salem R, Carr B et al (2004) Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 127:S194–S205
Lau W, Leung W, Ho S et al (1994) Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer 70:994–999
Lau W, Ho S, Leung T et al (1998) Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int J Radiat Oncol Biol Phys 40:583–592
Sangro B, Bilbao J, Boan J et al (2006) Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 66:792–800
Houle S, Yip T, Sheperd F et al (1989) Hepatocellular carcinoma: pilot trial of treatment with Y-90 microspheres. Radiology 172:857–860
Dancey J, Shepherd F, Paul K et al (2000) Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nucl Med 41:1673–1681
Carr B (2004) Hepatic arterial 90yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl 10(Suppl 2):S107–S110
Liu M, Uaje M, Al Ghazi M et al (2004) Use of yttrium-90 TheraSphere for the treatment of unresectable hepatocellular carcinoma. Am Surg 70:947–953
Salem R, Lewandowski R, Atassi B et al (2005) Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 16:1627–1639
Sato K, Lewandowski R, Bui J et al (2006) Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere®): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 29:522–529
Kulik L, Atassi B, Van Holsbeeck L et al (2006) Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 94:572–586
Therasse P, Arbuck S, Eisenhauer E et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Murthy R, Nunez R, Szklaruk J et al (2005) Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics 25(Suppl 1):S41–S55
Pöpperl G, Helmberger T, Münzing W et al (2005) Selective internal radiation therapy with SIR-Spheres® in patients with nonresectable liver tumors. Cancer Biother Radiopharm 20:200–208
Saar B, Kellner-Weldon F (2008) Radiological diagnosis of hepatocellular carcinoma. Liver Int 28:189–199
Salem R, Thurston K (2006) Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies part 2: special topics. J Vasc Interv Radiol 17:1425–1439
Coldwell D, Kennedy A (2005) Treatment of hepatic metastases from breast cancer with yttrium-90 SIR-Spheres radioembolization. Society of Interventional Radiology 2005 annual meeting, New Orleans, LA.
Goin J, Dancey J, Roberts C et al (2004) Comparison of post-embolization syndrome in the treatment of patients with unresectable hepatocellular carcinoma: trans-catheter arterial chemo-embolization versus yttrium-90 glass microspheres. World J Nucl Med 3:49–56
Murthy R, Brown D, Salem R et al (2007) Gastrointestinal complications associated with hepatic arterial yttrium-90 microsphere therapy. J Vasc Interv Radiol 18:553–562
Carretero C, Munoz-Navas M, Betes M et al (2007) Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol 102:1216–1220
Salem R, Thurston KG (2006) Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies part 1: technical and methodologic considerations. J Vasc Interv Radiol 17:1251–1278
Lewandowski R, Salem R (2004) Incidence of radiation cholecystitis in patients receiving Y-90 treatment for unresectable liver malignancies [Abstract]. J Vasc Interv Radiol 15:S162
Leung T, Lau W, Ho S et al (1995) Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys 33:919–924
Salem R, Lewandowski R, Sato K et al (2007) Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol 1:12–29
Neff R, Abdel-Misih R, Khatri J et al (2008) The toxicity of liver directed yttrium-90 micrcospheres in primary and metastatic liver tumors. Cancer Invest 26:173–177
Wollner I, Knutsen C, Ullrich K et al (1987) Effects of hepatic arterial yttrium-90 microsphere administration alone and combined with regional bromodeoxyuridine infusion in dogs. Cancer Res 47:3285–3290
Atassi B, Bangash A, Bahrani A et al (2008) Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics 28:81–99
Bienert M, McCook B, Carr B et al (2005) (90)Y microsphere treatment of unresectable liver metastases: changes in (18)F-FDG uptake and tumour size on PET/CT. Eur J Nucl Med Mol Imaging 32:778–787
Wong C, Qing F, Savin M et al (2005) Reduction of metastatic load to liver after intraarterial hepatic yttrium-90 radioembolization as evaluated by [18F]fluorodeoxyglucose positron emission tomographic imaging. J Vasc Interv Radiol 16:1101–1106
Kwee TC, Takahara T, Ochiai R, Nievelstein RAJ, Luijten PR et al (2008) Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol 18:1937–1952. doi:10.1007/s00330-008-0968-z
US National Institutes of Health (NIH) Medical Research Center. http://clinicalresearch.nih.gov/. Accessed 1 May 2008
Bangash A, Atassi B, Kaklamani V et al (2007) 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol 18:621–628
Coldwell D, Kennedy A, Nutting C (2007) Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys 69:800–804
Gulec S, Mesoloras G, Dezarn W et al (2007) Safety and efficacy of Y-90 microsphere treatment in patients with primary and metastatic liver cancer: the tumor selectivity of the treatment as a function of tumor to liver flow ratio. J Transl Med 5:15–23
Llovet S, Ricci V, Mazzaferro P et al (2007) Sorafenib improves survival in advanced hepatocellular carcinoma (HCC): results of a phase III randomized placebo-controlled trial (SHARP trial) [Abstract]. J Clin Oncol 25:18S
Hochster H, Hart R, Hainsworth J et al (2006) Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): final analysis of the TREE-study [Abstract]. J Clin Oncol 24:148s
Hochster H, Welles L, Hart R et al (2005) Safety and efficacy of bevacizumab (Bev) when added to oxaliplatin/fluoropyrimidine (O/F) regimens as first-line treatment of metastatic colorectal cancer (mCRC): TREE 1 & 2 Studies [Abstract]. J Clin Oncol 23:249s
Kennedy A (2007) 90Y microspheres + ChemoTx first line patients with mCRC. Presented at the emerging trends in radioembolisation using microspheres, clinical workshop, Chicago, IL, 4–5 May 2007
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Vente, M.A.D., Wondergem, M., van der Tweel, I. et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 19, 951–959 (2009). https://doi.org/10.1007/s00330-008-1211-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1211-7