Abstract
Purpose
Angiogenesis inhibition has emerged as a potentially promising treatment strategy for neuroendocrine tumors. 2-Methoxyestradiol (2ME2; Panzem®) is a natural derivative of estradiol with demonstrated anti-angiogenic activity in animal models. We performed a prospective, phase II study of 2ME2, administered in combination with bevacizumab, in patients with advanced carcinoid tumors.
Methods
Thirty-one patients with advanced carcinoid tumors were treated with 2ME2, administered orally at a dose of 1,000 mg four times daily. Patients also received bevacizumab 5 mg/kg intravenously every 2 weeks. Patients were observed for evidence of toxicity, tumor response, and survival.
Results
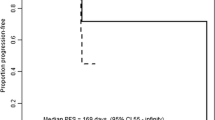
The combination of 2ME2 and bevacizumab was relatively easily tolerated and was associated with anticipated toxicities for these two agents. No confirmed radiologic responses (by RECIST) were observed. However, 68% of the radiologically evaluable patients experienced at least some degree of tumor reduction, and the median progression-free survival (PFS) time was 11.3 months.
Conclusion
2ME2 and bevacizumab can be safely administered to patients with advanced carcinoid tumors. While major tumor regression was not observed with this regimen, the encouraging median progression-free survival time suggests that this regimen has some degree of antitumor activity and supports the further investigation of angiogenesis inhibitors in this disease.


Similar content being viewed by others
References
Moertel C, Lefkopoulo M, Lipsitz S et al (1992) Streptozocin-doxorubicin, stretpozocin-fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 326:519–523
Kulke M, Hornick J, Frauenhoffer C et al (2009) O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 15:338–345
Ekeblad S, Sundin A, Janson ET et al (2007) Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 13:2986–2991
Arnold R, Muller H, Schade-Brittinger C et al (2009) Placebo-controlled, double blind, prospective, randomized study of the efect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. 2009 Gastrointestinal Cancers Symposium A121
Faiss S, Pape UF, Bohmig M et al (2003) Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors–the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 21:2689–2696
Zhang J, Jia Z, Li Q et al (2007) Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 109:1478–1486
Terris B, Scoazec J, Rubbia L (1998) Expression of vascular endothelial growth factor in digestive neuroendocrine tumors. Histopathology 32:133–138
Hobday TJ, Rubin J, Holen K et al (2007) MC044 h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): a Phase II Consortium (P2C) study (abstract). J of Clin Onc ASCO Annual Meeting Proc Part 1 25:4504
Kulke MH, Lenz HJ, Meropol NJ et al (2008) Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 26:3403–3410
Yao JC, Phan A, Hoff PM et al (2008) Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 26:1316–1323
Hughes RA, Harris T, Altmann E et al (2002) 2-Methoxyestradiol and analogs as novel antiproliferative agents: analysis of three-dimensional quantitative structure-activity relationships for DNA synthesis inhibition and estrogen receptor binding. Mol Pharmacol 61:1053–1069
Fotsis T, Zhang Y, Pepper MS et al (1994) The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 368:237–239
Becker CM, Rohwer N, Funakoshi T et al (2008) 2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol 172:534–544
Dahut WL, Lakhani NJ, Gulley JL et al (2006) Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther 5:22–27
Sweeney C, Liu G, Yiannoutsos C et al (2005) A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res 11:6625–6633
Liu G, sidor C, Feierabend C et al (2005) Phase Ib trial of 2ME2 administered as a nanocrystal dispersion (NCD) in patients with advanced cancer. Proceedings of EORTC/AACR/NCI B14:129
Sweeney C, Slebe K, Li M et al (2005) A single center open label dose escalation safety and pharmacokinetic study of 2 methoxyestriadiol nanocrystal colloidal dispersion in patients with advanced cancer. Proceedings of EORTC/AACR/NCI B12:157
Matei D, Schilder J, Sutton G et al (2009) Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: a Hoosier Oncology Group trial. Gynecol Oncol 115:90–96
Schumacher G, Kataoka M, Roth JA et al (1999) Potent antitumor activity of 2-methoxyestradiol in human pancreatic cancer cell lines. Clin Cancer Res 5:493–499
Sosman J, Puzanov I (2009) Combination targeted therapy in advanced renal cell carcinoma. Cancer 115:2368–2375
Feldman DR, Baum MS, Ginsberg MS et al (2009) Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 27:1432–1439. [see comment]
Korse CM, Bonfrer JM, Aaronson NK et al (2009) Chromogranin A as an alternative to 5-hydroxyindoleacetic acid in the evaluation of symptoms during treatment of patients with neuroendocrine Tumors. Neuroendocrinology 89:296–301
Yao JC, Phan AT, Chang DZ et al (2008) Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. [erratum appears in J Clin Oncol. 2008 Dec 1;26(34):5660] J Clin Oncol 26:4311–4318
Raymond E (2009) Phase III randomised double blind trial of suntinib versus placebo in patients with progressive, well-differentiated pancreatic neuroendocrine tumors. World Congress on Gastrointestinal Cancers, Barcelona, Spain
Kulke MH, Bergsland EK, Ryan DP et al (2006) Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J Clin Oncol 24:3555–3561
Duran I, Kortmansky J, Singh D et al (2006) A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer 95:1148–1154
Yao JC, Zhang JX, Rashid A et al (2007) Clinical and in vitro studies of imatinib in advanced carcinoid tumors. Clin Cancer Res 13:234–240
Acknowledgments
Support for this study was provided by Entremed, Inc, NCI grants CA093401 (MHK) and P50 CA127003 (DF/HCC SPORE in Gastrointestinal Cancer). The authors gratefully acknowledge support from the Saul and Gitta Kurlat fund for neuroendocrine tumor research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reported in part at the American Society of Clinical Oncology 2008 Gastrointestinal Cancers Symposium.
Rights and permissions
About this article
Cite this article
Kulke, M.H., Chan, J.A., Meyerhardt, J.A. et al. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol 68, 293–300 (2011). https://doi.org/10.1007/s00280-010-1478-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1478-7