Abstract
Purpose
To compare retrospectively the safety and efficacy of percutaneous and surgical implantations of port-catheters for intra-arterial hepatic chemotherapy (IAHC).
Materials and Methods
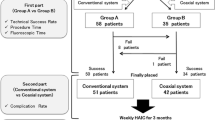
Between January 2004 and December 2008, 126 consecutive patients (mean age 58 years) suffering from liver colorectal metastases were referred for intra-arterial hepatic chemotherapy (IAHC). Port-catheters were percutaneously implanted (P) through femoral access with the patient under conscious sedation when no other surgery was planned or were surgically implanted (S) when laparotomy was performed for another purpose. We report the implantation success rate, primary functionality, functionality after revision, and complications of IAHC.
Results
The success rates of implantation were 97% (n = 65 of 67) for P and 98% (n = 58 of 59) for S. One hundred eleven patients received IAHC in our institution (n = 56P and n = 55S). Primary functionality was the same for P and S (4.80 vs. 4.82 courses), but functionality after revision was significantly higher for P (9.18 vs. 5.95 courses, p = 0.004) than for S. Forty-five complications occurred during 516 courses for P and 28 complications occurred during 331 courses for S. The rates of discontinuation of IAHC linked to complications of the port-catheters were 21% (n = 12 of 56) for P and 34% (n = 19 of 55) for S.
Conclusion
Overall, significantly better functionality and similar complication rates occurred after P versus S port-catheters.
Similar content being viewed by others
Introduction
Systemic chemotherapy for patients with from colorectal liver metastases has improved considerably. During the past 10 years, the use of new chemotherapeutic drugs (such as irinotecan and oxaliplatin) and, more recently, targeted therapies (such as bevacizumab and cetuximab) has improved the response rate of liver metastases and, more importantly, patient survival [1–4]. To continue to improve the efficacy of chemotherapy and decrease its toxicity and side effects to untargeted organs, many attempts have been made to deliver drugs directly to the liver by way of the arterial supply rather than into the systemic circulation.
Intra-arterial hepatic chemotherapy (IAHC) is administered by way of a catheter inserted into the hepatic artery and connected to a subcutaneously implanted port. Until recently, the implantation of these arterial port-catheters required laparotomy; however, recent advances in minimally invasive techniques have allowed their percutaneous placement [5]. The purpose of our study was to retrospectively compare the safety and efficacy of percutaneous and surgical implantation of port-catheters for IAHC.
Materials and Methods
Patients
We retrospectively analyzed 126 consecutive patients who were selected for IAHC in our institution from January 2004 to December 2008. The inclusion criteria were either unresectable liver colorectal metastases or adjuvant treatment after curative treatment of liver colorectal metastases. There was no evidence of extrahepatic disease on imaging work-up performed within 4 weeks before the multidisciplinary meeting that decided to implant the arterial port-catheter. This imaging work-up included at least chest and abdomino-pelvic computed tomography.
Port-catheters were either percutaneously implanted (P) through femoral access when no surgery was planned or were implanted surgically (S) when laparotomy was performed for another purpose, such as resection of colorectal cancer or liver colorectal metastases. Patients were followed-up from the date of the catheter placement until the discontinuation of IAHC.
Catheter Implantation
Percutaneous Implantation of the Port-Catheter
Percutaneous implantation of the port-catheter was performed by an interventional radiologist. Immediately before and throughout the procedure, patients were administered minimal conscious sedation and local anesthesia with 1% lidocaine. First, a 5F cobra-shaped catheter (Cook, Bjaeverskov, Denmark) was inserted in the femoral artery. Diagnostic mesenteric and celiac global angiograms were obtained to assess the arterial supply to the liver. If a replaced hepatic artery was present, embolization was performed with 0.018- or 0.035-inch steel coils (Tornado; Cook) to allow perfusion of the entire liver through the proper hepatic artery. If the right gastric and supraduodenal arteries were identified, they were embolized with 0.018-inch steel coils (Tornado) after catheterization with a 2.4F microcatheter (Progreat; Terumo, Tokyo, Japan).
Then a long, tapered 5F catheter (Anthron PU; Toray Industries, Tokyo, Japan) was used as an indwelling catheter and inserted over an 0.018-inch guidewire (V-18 Control Wire; Boston Scientific, Miami, FL) through the right femoral artery into the gastroduodenal artery by way of the celiac trunk. The side hole was located at the distal portion of the common hepatic artery just before the gastroduodenal artery, and the catheter tip was “fixed” [6] to the gastroduodenal artery with 0.018-inch coils. To avoid puncturing the controlateral femoral artery, this embolization was performed with a 2.4F microcatheter (Progreat) inserted into the indwelling catheter and exiting by way of its side hole to reach the gastroduodenal artery.
Finally, the catheter was connected through a subcutaneous tunnel to a subcutaneously implanted port (Celsite ST-305C; B. Braun Medical, Center Valley, PA) through a longitudinal skin incision performed 2 cm medial to the antero-superior iliac crest. Port-catheter implantation was considered technically successful if the contrast medium injected through the port entirely opacified the liver without any extrahepatic perfusion. Patients were asked to remain in bed for 4 h after the procedure.
Surgical Implantation of the Port-Catheter
Surgical implantation of the port-catheter was performed by a surgeon [7]. During laparotomy, the proximal part of the gastroduodenal artery was dissected a few centimeters downstream of the origin of the gastroduodenal artery. The indwelling catheter was inserted proximally in a retrograde fashion, by longitudinal arteriotomy, until its tip was exactly positioned in the orifice of the gastroduodenal artery. The catheter tip was secured with nonresorbable sutures. If a replaced hepatic artery was present, it was occluded to allow perfusion of the entire liver through the common hepatic artery: A left hepatic artery coming from the left gastric artery was ligated during the same procedure, whereas a right hepatic artery coming from the superior mesenteric artery was presurgically embolized with 0.018- or 0.035-inch steel coils by an interventional radiologist. Port-catheter implantation was considered technically successful if the entire liver parenchyma turned green after injection of 5 ml fluorescein through the indwelling catheter. Then the right gastric artery was ligated, as were all accessory vessels distal to the site of catheter placement, to prevent misperfusion of the upper gastrointestinal tract. Finally, the port (Celsite T202F; B. Braun Medical) was implanted in a subcutaneous pocket created in the front of the right lower ribs.
IAHC
The functionality of the port-catheters was evaluated by the number of IAHC courses that could successfully be delivered through it until discontinuation of treatment. The IAHC regimen [8] combined 100 mg oxaliplatin perfused over 2 h in the arterial port-catheter and systemic chemotherapy with 5-fluorouracil and leucovorin delivered over 48 h (simplified LVFU2 regimen, i.e., 200 mg m² leucovorin and 5-fluorouracil [bolus 400 and 1200 mg m², respectively, in a continuous perfusion over 46 hours]). All courses of IAHC were performed every 2 weeks by the same medical oncology team.
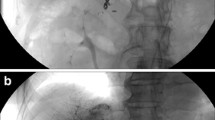
The port-catheters were systematically checked before every course of IAHC using anterior–posterior projection digital-subtracted angiogram with a frame rate of around 4 frames per second and manual injection of contrast medium through the port (Fig. 1). The goal of this “control angiogram” was to depict any complications that contraindicated the perfusion of IAHC.
A Control angiogram of a P port-catheter. The catheter tip (black arrow) is located in the gastroduodenal artery with coils around it, and the side hole is located in the common hepatic artery (white arrow). The right gastric artery is embolized with coils (arrowhead). B Control angiogram of an S port-catheter. The catheter tip (black arrow) is positioned in the orifice of the gastroduodenal artery and secured with nonresorbable sutures
When complications―such as extrahepatic perfusion, incomplete hepatic perfusion, catheter occlusion not responding to fibrinolysis (60,000 UI streptokinase perfused through the arterial port-catheter over 6 hours), or migration of the catheter tip―occured, the revision of the port-catheter was attempted by an interventional radiologist as follows:
-
1.
In patients with extrahepatic perfusion, the vessel involved was embolized with a microcatheter (Progreat) and 0.018-inch coils (Fig. 2).
-
2.
In patients with incomplete hepatic perfusion, the replaced hepatic artery was occluded with 0.018- or 0.035-inch coils to allow perfusion of the entire liver through the common hepatic artery (Fig. 3).
Fig. 3 A Incomplete hepatic perfusion depicted on control angiogram performed through the port-catheter. B Angiogram in a right hepatic artery associated with the common hepatic artery and not depicted during the surgical implantation of the port. C Embolization the right hepatic artery with 0.035-inch steel coils. D Perfusion of the entire liver through the common hepatic artery on control angiogram performed through the port-catheter
-
3.
In patients with catheter occlusion not responding to fibrinolysis, or in case of migration of the catheter tip, exchanges of the catheters were performed for P, but not for S, because these catheters are secured with nonresorbable sutures in the gastroduodenal artery and thus cannot be removed.
When complications―such as dissection or thrombosis of the hepatic artery, infection of the port, or bleeding at the puncture site―occurred, IAHC was stopped and the port removed.
Data Analysis
We compared the baseline characteristics between the two populations. We reported the success rates of implantation for P and S and the mean delay between implantation for P and S and the first course of IAHC. We reported complications of the port-catheters, and we compared the complication rate per course for P and S. We compared the primary functionality and the functionality after revision for S and P, and we evaluated the benefit of percutaneous revision of the port-catheters by comparison between primary functionality and functionality after revision for P and for S. We reported the causes of discontinuation of IAHC, and we compared discontinuation rates linked to port-catheter complications for P and for S.
Efficacy of IAHC in term of response and survival rates was not evaluated in this study. The different tests used for the different comparisons are reported in the Tables 1 and 3. Statistical significance was considered at p < 0.05.
Results
Two interventional radiologists and two surgeons in a single institution implanted 123 port-catheters to treat 126 indications for IAHC. The success rates of implantation for P and S were 97% (n = 65 of 67) and 98% (n = 58 of 59), respectively. The cause of P failure were stenosis of the celiac trunk (n = 2). The cause of S failure was inability to insert the catheter into the right hepatic artery, which was the only artery supplying the liver. S was performed in addition to resection of colorectal tumor (n = 20), resection of liver colorectal metastases (n = 30), or both (n = 9).
Twelve patients received IAHC at another hospital and could not be analyzed, namely due to variation in the evaluation of the catheter before each course and various regimen of chemotherapy. One hundred eleven patients received chemotherapy at our institution, including 56 P and 55 S procedures, and could be analyzed, (Table 1). Baseline characteristics between these two populations were similar with respect to age and sex, but higher rates of palliative treatment and a higher number of previous chemotherapy lines indicated a rather unfavorable prognosis for patients undergoing P. Such patients received their first course of IAHC sooner after implantation of the port-catheter than did S patients (13.7 vs. 50.5 days, respectively; p = 0.004. Primary functionality was not statistically significant (4.80 courses for P and 4.82 courses for S, p = 0.99). Functionality after revision was 9.18 courses for P (514 courses delivered in 56 patients) and 5.95 courses for S (327 courses delivered in 55 patients) (p = 0.004).
Different types of complications of the port-catheter are listed in Table 2. Rates of complications per course of IAHC were similar between P and S (p = 0.9): 45 complications―including thrombosis of the hepatic artery (n = 1), port infection (n = 2), bleeding at the puncture site (n = 3), extrahepatic perfusion (n = 20), incomplete hepatic perfusion (n = 6), occlusion of the catheter not responding to fibrinolysis (n = 1), and migration of the catheter tip (n = 8)―occurred in 33 patients during 516 P courses, and 28 complications―including dissection of the hepatic artery (n = 7), port infection (n = 2), extrahepatic perfusion (n = 5), incomplete hepatic perfusion (n = 9), and occlusion of the catheter not responding to fibrinolysis (n = 4)―occurred in 25 patients during 331 S courses. One S patient died from hepatic artery dissection.
The rate of extrahepatic perfusion per course (p = 0.04) and the rate of migration of the catheter tip per course (p = 0.005) were significantly higher for P than for S. The rate of occlusion of the catheter not responding to fibrinolysis was significantly higher for S than for P (p = 0.006).
The revisions significantly improved the functionality of P from 4.80 to 9.18 courses (p = 0.001) and of S from 4.82 to 5.95 courses (p = 0.001). The revisions were performed in 46 patients (n = 33 P and n = 13 S) for extrahepatic perfusion (n = 19 P and n = 5 S), incomplete hepatic perfusion (n = 5 P and n = 8 S), occlusion of the catheter not responding to fibrinolysis (n = 1 P and n = 0 S), and migration of the catheter tip (n = 8 P and n = 0 S).
Reasons for and rates of discontinuation of IAHS are listed in Table 3. The rates of discontinuation linked to complications of the port-catheters were 21% for P and 34% for S (p = 0.12). Other reasons for discontinuation of IAHC included tumor progression or death in 57% (n = 32 of 56) for P and 26% (n = 4 of 55) for S; chemotherapy-induced systemic toxicity in 7% (n = 4 of 56) for P and 25% (n = 14 of 55) for S (a response that allowed hepatic surgery or radiofrequency ablation in 9% [n = 5 of 56] for P and 9% [n = 5 of 55] for S); or completion of the planned adjuvant treatment in 5% (n = 3 of 56) for P and 5% (n = 3 of 55) for S.
Discussion
To further improve the efficacy of chemotherapy and decrease the toxicity and side effects to untargeted organs, many attempts have been made to deliver drugs directly to the liver through the arterial supply rather then into the systemic circulation. IAHC requires the use of catheters inserted into the hepatic artery using a subcutaneous arterial port-catheter. There are two different approaches for the placement of these ports: S or P. An obvious advantage of the P approach is to avoid invasive surgery when it is otherwise not warranted. Indeed, the P approach avoids the specific complications of laparotomy (which are not evaluated or reported here) and can be performed using minimal conscious sedation and local anesthesia. Furthermore, the first course of IAHC can be performed quickly after P, whereas it should be delayed after laparotomy because of the convalescence period. Overall, we demonstrated that primary functionality was the same for P and S but that functionality after revision was significantly higher for P than for S. Technical successes were high and were comparable for P and S, and the rates of complication were similar between P and S. Consequently, it can be advised to avoid S when surgery is not needed for other purpose.
Few studies in the literature compare the S and P approaches. Hildebrandt et al. [9] compared the complication and port duration between 41 P and 40 S procedures. Similar to our study, they report a high success rate for P (100%, n = 41 of 41) and S (95%, n = 38 of 40) and no difference in complication rates between P and S. Oberfield et al. [10] compared 42 P and 58 S procedures: The tumor response rates showed no significant difference between P (48%) and S (34%, p = 0.22), no significant difference in median survival time from treatment until death between P (1.06 month) and S (13 months, p = 0.39), and longer duration for P (19 months) than for S (14 months, p = 0.01).
The benefit of the interventional revision is obvious: It results in a significant increase of functionality for both P and S. This requires an interventional radiology team that systematically checks the port-catheters before each course of IAHC to depict and manage any complication. The gain after revision was significantly higher for P than for S (4.38 vs. 1.13 courses, p = 0.0006). This is probably because revisions are not possible due to some of the complications of S, namely, occlusion of the catheter not responding to fibrinolysis, which accounted for 14% of S complication. In this situation, exchange of catheter was not possible for S because these catheters were fixed by suture within the gastroduodenal artery and thus could not be removed. However, most P complications can be managed by interventional revision, thus increasing P functionality, whereas S complications were not fixable. Moreover, in cases of S complication, a second port can be placed percutaneously. In these cases, because the gastroduodenal artery was surgically ligated, the catheter tip is inserted as distally as possible in a branch of the hepatic artery, and the side hole is located in the common hepatic artery.
The major limitation of our study was the heterogeneous population. Indeed the proportion of patients who had undergone IAHC as adjuvant treatment was significantly lower for P (n = 3 of 56) than for S (n = 27 of 55, p < 0.0001). This probably indicates a rather unfavorable prognosis for P in terms of response. Due to these differences, it does not seem appropriate to compare response or survival rates. Despite heterogeneity, it is important to underline treatment consistency: All patients received follow-up by the same oncology team and consequently underwent the same IAHC drug regimen and the same modalities to evaluate functionality.
Conclusion
The P approach is a safe and efficient alternative to the S approach when no laparotomy is planned. The benefits of the interventional revision are significant and obvious, both for P and for S.
References
Saltz L, Clarke S, Díaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Kabbinavar FF, Hambleton J, Mass RD et al (2005) Combined analysis of efficacy: the addition of bevacizumab to fluorouracil of leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 23:3706–3712
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Giantonio BJ, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Herrmann KA, Waggershauser T, Sittek H et al (2000) Liver intraarterial chemotherapy: use of the femoral artery for percutaneous implantation of catheter-port systems. Radiology 215(1):294–299
Arai Y, Takeuchi Y, Inaba Y et al (2007) Interventional techniques for hepatic arterial infusion chemotherapy. Tech Vasc Interv Radiol 10:30–37
Elias D, de Baere T, Sideris L et al (2004) Regional chemotherapeutic techniques for liver tumors: current knowledge and future directions. Surg Clin North Am 84(2):607–625
Ducreux M, Ychou M, Laplanche A et al (2005) Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol 23:4881–4887
Hildebrandt B, Pech M, Nicolaou A et al (2007) Interventionally implanted port catheter systems for hepatic arterial infusion of chemotherapy in patients with colorectal liver metastases: a phase II-study and historical comparison with the surgical approach. BMC Cancer 7:69
Oberfield RA, Sampson E, Heatley GJ (2004) Hepatic artery infusion chemotherapy for metastatic colorectal cancer to the liver at the Lahey Clinic: comparison between two methods of treatment, surgical versus percutaneous catheter placement. Am J Clin Oncol 27(4):376–383
Acknowledgement
The authors of this paper wish to thank Joey Marie Robinson for her generous assistance in editing and preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors certify that there is no actual or potential conflict of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Deschamps, F., Elias, D., Goere, D. et al. Intra-Arterial Hepatic Chemotherapy: A Comparison of Percutaneous Versus Surgical Implantation of Port-Catheters. Cardiovasc Intervent Radiol 34, 973–979 (2011). https://doi.org/10.1007/s00270-010-9996-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-9996-6