Abstract
Background
In order to investigate the value of FDG positron emission tomography-computed tomography (PET-CT), FDG PET (reviewed side-by-side with CT), and metabolic parameters in the assessment of lymph node status and prognosis.
Methods
Fifty-five subjects with lymph node positive (N1) and 26 subjects with lymph node negative (N0) disease were included. In the slice with the maximum FDG uptake of the tumor, the axial area of the primary tumor, the maximum diameter of the tumor, and the mean and maximum standardized uptake values were measured.
Results
Fused PET-CT correctly characterized 289 of 325 lymph node groups (accuracy 89%) compared to 273 of 325 with PET (accuracy 84%). In lymph node staging (N0 vs. N1), PET-CT (accuracy 83%) was more accurate than PET (accuracy 78%). Among the metabolic parameters, the tumor diameter measured on PET-CT was the best predictor of lymph node stage (N0 vs. N1: accuracy 86%; threshold 25.5 mm) and overall survival. However, the highest accuracy of lymph node staging (N0 vs. N1) was achieved with the synergistic combination of visual analysis and primary tumor diameter measurements (accuracy 95%).
Conclusions
PET-CT increases accuracy of lymph node staging in esophageal adenocarcinoma compared to PET. The primary tumor diameter further improves accuracy in lymph node staging and was shown to be an independent predictor of overall survival.
Similar content being viewed by others
Lymph node status is considered the most important prognostic factor in patients with esophageal carcinoma [1–3]. The 5-year survival in lymph node negative disease was reported to be 42%–72% compared to 10%–12% in lymph node positive disease [4]. For overall staging of esophageal tumors, positron emission tomography-computed tomography (PET-CT) and PET are considered the most accurate modalities [5–7]. However, the sensitivity of PET in predicting lymph node positive disease based on visual analysis was shown to be lower than with endoscopic ultrasound [8]. This relatively low sensitivity has prevented PET from being the first choice modality in lymph node staging in esophageal carcinoma. In the present study, we investigated the additional value of fused PET-CT over PET reviewed side-by-side with CT in lymph node staging. Moreover, it is well established that increasing depth of tumor invasion is associated with higher risk of regional lymph node metastasis (N1). Deeper invasion into the esophageal wall and larger axial dimension of the primary tumor increases exposure to lymphatics and increases the potential risk of tumor spread to regional lymph nodes [9–13]. To the best of our knowledge, it has not been evaluated previously whether axial dimension of the primary tumor assessed with PET, or PET-CT is associated with lymph node status (N0 vs. N1) in esophageal carcinoma. Therefore, in the present study we measured tumor-width parameters including axial tumor area, maximum tumor diameter, and the standardized uptake value (SUV) in the slice with the maximum FDG uptake of the tumor. We investigated whether these tumor-width parameters are associated with lymphatic spread and whether they can predict N stage.
Patients and methods
Study population
Approval from our Institutional Review Board was received to undertake this retrospective Health Insurance Portability and Accountability Act—compliant study. The study group consisted of 81 consecutive patients who were found to have esophageal lesions with increased FDG uptake in pre-treatment PET-CT examinations. Table 1 shows that there was no difference between the 26 patients with N0 and the 55 patients with N1 disease in factors that might influence measurements of tumor-width parameters. Grading and typing of all tumors was done by histological examination, and complete TNM staging was performed according to the AJCC (American Joint Committee on Cancer) criteria. In 42 patients who received neoadjuvant chemoradiotherapy, the reference modalities for lymph node staging (N stage) were endoscopic ultrasound with fine-needle aspiration. In 39 patients who underwent primary surgery without neoadjuvant chemoradiotherapy, the reference standard was the postsurgical pathology. These 39 patients with a primary surgical approach underwent extensive lymphadenectomy as part of a left thoracoabdominal esophagectomy for low-lying lesions, and an Ivor Lewis esophagectomy for higher lesions. During surgery, lymph nodes were sampled, and the number, size, and precise location of the nodes were recorded according to a commonly used nodal station scheme [15]. Then, the presumed lymph nodes on the preoperative PET-CT scans were correlated with surgical and pathologic results for each nodal group. Patients did not have previous or secondary malignancy and had not undergone chemotherapy, radiotherapy, or endoscopic laser therapy prior to PET-CT scanning. Subjects with diabetes mellitus were not included. An electronic database of medical records was reviewed retrospectively to gather the above-mentioned clinical data of the patients. Patient data were documented in a database run by a statistical software program (SPSS, version 15.0, SPSS Inc, Chicago, IL, USA).
Data acquisition
18F-FDG PET-CT scans were obtained with a hybrid 3D PET-CT system (Biograph Sensation 16; Siemens, Erlangen, Germany) encompassing a 16-slice CT scanner and a PET system with lutetium oxyorthosilicate detectors. First, 555 MBq (15 mCi) of FDG was administered intravenously 60 min prior to the PET-CT scan. Patients were asked to fast for at least 6 h before FDG injection. A low-dose CT from skull to the mid-thigh was performed for attenuation correction. The CT component was obtained with a tube current of 150 mA, a tube voltage of 120 keV, rotational speed of 0.5 s, and a table feed of 18 mm per rotation. PET images were acquired in seven acquisition beds per patient and were reconstructed with CT-derived attenuation correction. Subsequently, diagnostic CT images were acquired by injecting 100 mL of 300-mg iodine/mL together with 20 mL of saline at 2 mL/s and a 60-s postinjection delay. Table feed of the diagnostic CT was 15 mm/s, the pitch was 1.5, the effective current-time product was 170 mAs, and the tube voltage was 140 kVp.
Histopathologic lymph node evaluation
Out of the 81 study subjects, 39 underwent primary surgery without neoadjuvant therapy. From these 39 patients a total of 325 lymph node groups were sampled, precisely localized according to a commonly used nodal station scheme [15], and histopathologically evaluated. Seventy-nine out of the 325 lymph node groups were shown to be malignant. These 79 malignant lymph node groups consisted of 3 right upper paratracheal lymph node groups, 1 posterior mediastinal, 0 right lower paratracheal, 3 subcarinal, 12 middle, 16 lower paraesophageal, 2 pulmonary ligament, 5 right tracheobronchial, 9 diaphragmatic, 3 paracardial, 12 left gastric, and 13 common hepatic lymph node groups.
Lymph node staging based on a visual analysis on PET and on PET-CT
Two radiologists (each with 4 years of experience in PET-CT reading) were blinded to the clinical data of the patients and performed the visual interpretation independently. First, lymph nodes were evaluated based on a visual analysis on PET reviewed side-by-side with CT followed by an analysis on combined PET-CT. FDG uptake in a presumed lymph node that was focally prominent compared with surrounding tissues was considered to be positive for malignancy. Both readers documented the presence, number, and precise location of malignant lymph nodes. In case of a discrepancy between both interpreters, a third reader was consulted to make a final decision.
Primary tumor width measurements for lymph node staging
All measurements were performed in the slice with the maximum FDG uptake of the tumor. The following tumor-width parameters were measured by the two readers separately: First, the PET area of the tumor was assessed on the PET-CT images. The PET area was delineated automatically by an isocontour with an SUV of 2.5 (outer circle in Fig. 1B, C) in the slice with the maximum FDG uptake of the tumor. This means that the PET area was delineated automatically by a circle encompassing pixels equal or greater than SUV 2.5 (outer circle in Fig. 1B, C) without considering information from the CT component. In some cases, the PET area (as defined with an SUV of 2.5) might, however, extend beyond the esophageal wall (as visible on CT) into adjacent lung tissue. This is demonstrated in Fig. 1B, C (arrow). Therefore, following the PET area delineation, the PET-CT area (inner circle in Fig. 1B, C) was determined to correct the PET area measurements by additionally considering the information from the CT component: the PET-CT area was defined by the outer wall of the esophagus (visible on the CT component), if the previously assessed PET area extended beyond the anatomic borders of the esophagus. The PET-CT area was equal to the PET area if the previously assessed PET area was within the esophageal walls visible on the CT component (Fig. 2B, C). The PET and PET-CT diameters represent the maximum diameter of the PET and PET-CT area, respectively. Maximum SUV (SUV max) and mean SUVs in both PET (SUV of area, PET) and PET-CT area (SUV of area, PET-CT) were automatically measured and normalized to body weight. In all measurements, average values were calculated from the results of both readers. These mean values were used for statistical analysis.
Example of PET-CT diameter and PET diameter measurements in a patient with lymph node positive (N1) disease of esophageal cancer. Demonstrated are images of PET alone (A), fused PET-CT (B), and CT alone (C). Tumor diameter measurements were performed in the slice with the maximum FDG uptake of the tumor. Results: PET area was automatically defined by an SUV isocontour of 2.5 (outer circle, B, C): 834 mm2; PET diameter: 36.1 mm; mean SUV of PET area: 4.9. PET-CT area was defined by the outer esophageal wall visible in the CT component (inner circle, B, C): 546 mm2; PET-CT diameter: 30.5 mm, mean SUV of PET-CT: 5.8.
Example of PET-CT diameter and PET diameter measurements in a patient with lymph node negative (N0) disease of esophageal cancer. Demonstrated are images of PET alone (A), fused PET-CT (B), and CT alone (C). Tumor diameter measurements were performed in the slice with the maximum FDG uptake of the tumor. In this example, PET-CT area was equal to PET area, because PET area (defined by SUV isocontour of 2.5) was within the anatomic boundaries of the esophageal wall. Measurements: PET and PET-CT area (circle B, C): 109 mm2; PET-CT and PET diameter: 12.1 mm; mean SUV of PET and PET-CT area: 3.9.
Data analysis and statistics
All statistical tests were two-sided and performed at the 5% level of significance by using SPSS for Windows, version 15.0 (SPSS Inc, Chicago, IL, USA). Differences in patient and tumor characteristics, differences in tumor area, diameter, and SUV were evaluated with Chi-square and t-tests. Binary logistic regression analysis with a stepwise approach determined which of the tumor-width parameters was most strongly associated with the N-stage. Kaplan–Meier curves, Log-Rank test, and Cox regression analysis were performed for survival analysis.
Results
Analysis of lymph node groups: sensitivity of PET-CT and PET in a visual interpretation
Table 2 shows that PET-CT had higher sensitivity in identifying lymph node groups than PET in a side-by-side analysis with CT. With PET-CT, 55 out of the 79 (70%) malignant lymph node groups were accurately characterized, whereas only 49 out of the 79 (62%) malignant node groups were detected by PET in a side-by-side analysis with CT (Table 2). Thirty nodal groups had false negative findings on PET. Six of these were correctly shown to have metastasis on PET-CT. These included two middle, and three lower paraesophageal lymph node groups with diameters of 6–11 mm, and one left gastric lymph node with a diameter of 6 mm. The 24 false negative lymph node groups that were missed both on PET and PET-CT included six middle and nine lower paraesophageal lymph node groups with a mean lymph node diameter of 5 mm, three common hepatic (mean diameter 5 mm), two left gastric (mean diameter 4 mm), two diaphragmatic (mean diameter 5 mm), one right tracheobronchial (diameter 4 mm), and one paracardial node groups (diameter 5 mm).
Analysis of lymph node groups: specificity of PET-CT and PET in a visual interpretation
PET-CT was more specific (95%) than PET (91%) in diagnosing lymph node groups (Table 2). PET-CT correctly characterized 10 of the 22 false positive findings on PET as benign uptake. These consisted of four lesions that were interpreted on PET as malignant lymph nodes adjacent to the primary tumor, but demonstrated to be part of the inhomogenous tumor on PET-CT. Furthermore, six false positive findings on PET were precisely localized and diagnosed on PET-CT as physiologic uptake of the stomach (n = 3) and the distal esophagus (n = 3). Twelve benign lymph node groups were falsely characterized as malignant both on PET-CT and PET including three posterior mediastinal, three right tracheobronchial, two right lower paratracheal, two diaphragmatic, one left gastric, and one common hepatic lymph node group. These 12 false positive lymph node groups were hyperplastic due to inflammation (n = 9) or due to granulomatous disease (n = 3) on histopathological evaluation.
Lymph node staging (N0 vs. N1) based on tumor-width parameters
Out of the 81 study subjects, 55 patients had lymph node positive (N1) and 26 patients had lymph node negative (N0) disease. In the slice of the maximum FDG uptake of the primary tumor, the tumor-width parameters including tumor diameter, tumor area, mean SUV, and maximum SUVs were measured on PET-CT and PET (Table 3). Each of these tumor-width parameters was increased in lymph node positive (N1) compared to lymph node negative disease (N0). As shown in Table 3, the tumor diameter was the most accurate tumor-width parameter for lymph node staging both on PET-CT and PET measurements. Using the optimal threshold of 25.5 mm (assessed in an ROC curve), the tumor diameter predicted N1 stage with a sensitivity of 87% and a specificity of 85%. The interrater consistency between both readers was determined in linear regression models with R 2 values of 0.987 for PET area, 0.983 for PET-CT area, 0.987 for PET diameter, 0.984 for PET-CT diameter, 0.992 for mean SUV of PET area, 0.989 for mean SUV of PET-CT area, and 0.986 for maximum SUV. Furthermore, there was excellent correlation between tumor diameter assessed on endoscopic ultrasound (EUS) and tumor diameter measured with PET-CT (r = 0.888, P < 0.001; Pearson correlation) and PET (r = 0.795, P < 0.001).
Lymph node staging (N0 vs. N1) based on the combination of visual analysis and tumor-width parameters
Table 4 demonstrates accuracies of PET-CT and PET in lymph node staging (N1 vs. N0) based on a visual analysis, based on tumor diameter measurements, and based on the combination of visual analysis and tumor diameter measurements. The quantitative approach (tumor diameter) was more sensitive, but less specific than the visual analysis. The highest accuracy (sensitivity: 95%, specificity: 96%, accuracy: 95%) was achieved when combining qualitative and quantitative analysis. The combination of visual analysis and quantitative analysis is explained in Table 5: first, images are interpreted visually and lymph node staging is performed. In the visual interpretation, the absence or presence of malignant appearing lymph nodes is documented with a score of 0 or 1, respectively. After that, the tumor diameter is assessed and a score of 1 is given, when the diameter is equal or greater than 37.8 mm for PET-CT or 29.8 mm for PET measurements (quantitative interpretation). For the combination with a visual analysis, we chose those higher thresholds of the diameters (37.8 and 29.8 mm for PET-CT and PET, respectively) to achieve higher specificity: a tumor diameter above these cut-offs indicates lymph node positive disease, even if the visual analysis detected no malignant appearing lymph nodes. This means that a tumor is classified as lymph node positive (combination Index ≥ 1, Table 4) when either an FDG avid lymph node can be identified on the visual analysis and/or when the diameter of the primary tumor is ≥37.8 mm on PET-CT (≥29.8 mm on PET, Table 5).
Predicting prognosis based on the tumor diameter
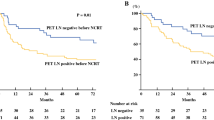
The tumor diameter measured on PET-CT was shown to be the most accurate predictor of survival. This was assessed in a Cox-regression analysis with inclusion of lymph node stage (N0 vs. N1), tumor diameter, tumor area, the maximum SUV, and the mean SUV. A PET-CT diameter of 27.5 mm was shown (in an ROC curve analysis) to be the optimal cut-off in predicting death during the follow-up period. Tumors with a diameter of <27.5 mm (n = 38) had a significantly longer mean survival time (33.2 months, 95% CI: 29.8–36.5) than patients with a tumor diameters of ≥27.5 mm (n = 43, mean survival: 15.5 months, 95% CI: 11.6–19.3; χ2: 28.3, P < 0.001, Log-Rank test).
Discussion
The lymph node status is the most important prognostic and a critical therapeutic factor of esophageal carcinoma [1–4, 10, 12]. Patients with lymph node negative disease (N0) are treated with primary surgical resection, whereas patients with N1 disease undergo neoadjuvant chemoradiotherapy before surgery. The present study evaluated the incremental value of PET-CT over PET in lymph node staging of adenocarcinomas of the esophagus. In our investigation, the advantage of fused PET-CT over PET in the characterization of lymph node could be explained by the structural map provided by CT, which improved the localization of tracer activity. Especially paraesophageal, FDG avid lymph nodes that appeared to be part of the primary tumor on PET could be spatially discriminated by the CT component and identified as lymph node metastasis on PET-CT. Lymph nodes measuring 6–11 mm that were false negative on PET could be identified on PET-CT. Lymph nodes that were missed on both PET-CT and PET were smaller than 6 mm. The specificity of PET-CT was higher than that of PET especially due to the exact localization of structures that are physiologically FDG avid including the esophagogastric junction and the gastric antrum. Besides PET-CT and PET, the current imaging techniques for the detection of lymph node involvement in esophageal carcinoma include EUS and CT. Previous studies have investigated the performance of PET in lymph node staging based on a visual analysis by evaluating the presence or absence of FDG positive lymph nodes. The low sensitivity of PET compared to EUS (33% vs. 81%, respectively) in these studies has so far limited the use of PET in lymph node staging of esophageal carcinoma [8]. Specificity, however, is better in PET compared to EUS (89% vs. 67%) [8], and even the combined results of EUS and CT failed to improve their cumulative specificity over that of PET in distant lymph node staging [16]. This demonstrates that despite its superior sensitivity, EUS cannot differentiate between benign and malignant lymph node enlargement. Several investigations have also compared CT with PET in lymph node staging. Specificities were similarly good in both modalities ranging from 78% to 100% for PET and from 73% to 100% for CT [5, 8, 17–22]. Overall sensitivities have been shown to be low both for PET and CT, but with superior results for PET compared to CT. A study by Kim et al. [18] found a sensitivity of 52% for PET and of 15% for CT in the detection of lymph node groups. These results for PET are consistent with a meta-analysis of the literature that found a sensitivity of 51% and a specificity of 84% for PET in lymph node staging of esophageal carcinoma [23]. In summary, EUS has high sensitivity, but low specificity, and both CT and PET demonstrate high specificity, but low sensitivity. The results of our study for PET are compliant with these previous results: PET with side-by-side review of CT had relatively low sensitivity (75%) and good specificity (85%) in lymph node staging (accuracy 78%). Combined PET-CT could not significantly increase sensitivity (76%), whereas specificity (95%) and accuracy (83%) could be improved compared to PET based on a visual analysis (qualitative). This is consistent with a previous report from lymph node staging in squamous cell carcinoma of the esophagus with integrated PET-CT, which showed an increase in accuracy by 6% on PET-CT compared to PET alone [7]. The relatively low sensitivity (in our study 76%) of a visual analysis hinders PET-CT from being the gold standard in lymph node staging. Therefore, it would be desirable if especially the sensitivity in PET-CT and PET for evaluation of lymph nodes could be further improved to enhance accuracy of PET-CT in clinical lymph node staging of esophageal cancer. In the present study, tumor diameter measurements with PET-CT yielded a sensitivity of 87% and specificity of 85% in predicting lymph node positive disease which makes this quantitative test more accurate (accuracy 86%) for lymph node staging than visual analysis with PET, PET-CT, EUS, or CT (their accuracies were discussed above). However, the highest accuracy for determining N stage (N0 vs. N1) was achieved in a combination of the qualitative (visual) and the quantitative (tumor diameter measurements) analysis with a sensitivity of 95% and specificity of 96% (accuracy 95%). This demonstrates the highly synergistic effect when combining the visual and quantitative analysis for lymph node staging. Hence, the relatively low sensitivity of PET-CT in determining lymph node status can be overcome by a complementary combination of visual analysis and tumor diameter measurements. Our study was based on previous observations that increased local tumor extension and depth of tumor invasion is predictive of regional lymph node metastasis (N1) in esophageal carcinoma [9–13]. This means that the further the primary tumor has progressed in axial dimension and has infiltrated the esophageal wall the higher the risk of lymphatic spread. Our study confirms that the axial dimension of the primary tumor (tumor diameter) as measured with PET-CT accurately predicts lymph node stage. Moreover, the diameter of the primary tumor independently predicts overall survival and was shown to be a more accurate prognostic factor than both the SUV and than the lymph node status (N0 vs. N1 stage). PET diameter and especially PET-CT diameter were strongly correlated with the tumor diameter measured on endoscopy. By determining the slice with the maximum FDG uptake, the PET component of the integrated PET-CT makes it possible to localize and measure the diameter of the metabolically most active and presumably the most advanced part of the tumor with a high interrater reliability.
In conclusion, fused PET-CT was more accurate in lymph node staging than PET in both a lesion-based analysis (lymph node groups) and in a patient-based analysis (N0 vs. N1). Furthermore, the diameter of the primary tumor determined on PET-CT was found to be an accurate predictor of lymph node stage and overall survival. The combination of a visual analysis and quantitative tumor diameter measurements yielded a higher accuracy in lymph node staging than visual and quantitative analysis alone.
References
de Manzoni G, Pedrazzani C, Pasini F, et al. (2003) Pattern of recurrence after surgery in adenocarcinoma of the gastro-oesophageal junction. Eur J Surg Oncol 29:506–510
Steup WH, De Leyn P, Deneffe G, et al. (1996) Tumors of the esophagogastric junction. Long-term survival in relation to the pattern of lymph node metastasis and a critical analysis of the accuracy or inaccuracy of pTNM classification. J Thorac Cardiovasc Surg 111:85–94
Rudiger SJ, Feith M, Werner M, et al. (2000) Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 232:353–361
Lerut T, Coosemans W, Decker G, et al. (2001) Cancer of the esophagus and gastro-esophageal junction: potentially curative therapies. Surg Oncol 10:113–122
Kato H, Miyazaki T, Nakajima M, et al. (2005) The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 103:148–156
Bar-Shalom R, Guralnik L, Tsalic M, et al. (2005) The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging 32:918–924
Yuan S, Yu Y, Chao KS, et al. (2006) Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med 47:1255–1259
Flamen P, Lerut A, Van Cutsem E, et al. (2000) Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 18:3202–3210
Westerterp M, Koppert LB, Buskens CJ, et al. (2005) Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 446:497–504
Hagen JA, DeMeester SR, Peters JH, et al. (2001) Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg 234:520–530
Nigro JJ, Hagen JA, DeMeester TR, et al. (1999) Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 117:16–23
Holscher AH, Bollschweiler E, Bumm R, et al. (1995) Prognostic factors of resected adenocarcinoma of the esophagus. Surgery 118:845–855
Riquet M, Saab M, Le Pimpec BF, et al. (1993) Lymphatic drainage of the esophagus in the adult. Surg Radiol Anat 15:209–211
Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85:1457–1459
Cerfolio RJ, Bryant AS, Ohja B, et al. (2005) The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 129:1232–1241
Lerut T, Flamen P, Ectors N, et al. (2000) Histopathologic validation of lymph node staging with FDG-PET scan in cancer of the esophagus and gastroesophageal junction: A prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg 232:743–752
Block MI, Patterson GA, Sundaresan RS, et al. (1997) Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 64:770–776
Kim K, Park SJ, Kim BT, et al. (2001) Evaluation of lymph node metastases in squamous cell carcinoma of the esophagus with positron emission tomography. Ann Thorac Surg 71:290–294
Rankin SC, Taylor H, Cook GJ, et al. (1998) Computed tomography and positron emission tomography in the pre-operative staging of oesophageal carcinoma. Clin Radiol 53:659–665
Rasanen JV, Sihvo EI, Knuuti MJ, et al. (2003) Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol 10:954–960
Yoon YC, Lee KS, Shim YM, et al. (2003) Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology 227:764–770
Flanagan FL, Dehdashti F, Siegel BA, et al. (1997) Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol 168:417–424
van Westreenen HL, Westerterp M, Bossuyt PM, et al. (2004) Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 22:3805–3812
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roedl, J.B., Blake, M.A., Holalkere, N.S. et al. Lymph node staging in esophageal adenocarcinoma with PET-CT based on a visual analysis and based on metabolic parameters. Abdom Imaging 34, 610–617 (2009). https://doi.org/10.1007/s00261-008-9447-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-008-9447-x