Abstract.
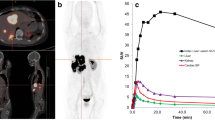
Recent advances in receptor-mediated tumour imaging have resulted in the development of a new somatostatin analogue, DOTA-dPhe1-Tyr3-octreotide. This new compound, named DOTATOC, has shown high affinity for somatostatin receptors, ease of labelling and stability with yttrium-90 and favourable biodistribution in animal models. The aim of this work was to evaluate the biodistribution and dosimetry of DOTATOC radiolabelled with indium-111, in anticipation of therapy trials with 90Y-DOTATOC in patients. Eighteen patients were injected with DOTATOC (10 µg), labelled with 150–185 MBq of 111In. Blood and urine samples were collected throughout the duration of the study (0–2 days). Planar and single-photon emission tomography images were acquired at 0.5, 3–4, 24 and 48 h and time-activity curves were obtained for organs and tumours. A compartmental model was used to determine the kinetic parameters for each organ. Dose calculations were performed according to the MIRD formalism. Specific activities of >37 GBq/ µmol were routinely achieved. Patients showed no acute or delayed adverse reactions. The residence time for 111In-DOTATOC in blood was 0.9±0.4 h. The injected activity excreted in the urine in the first 24 h was 73%±11%. The agent localized primarily in spleen, kidneys and liver. The residence times in source organs were: 2.2±1.8 h in spleen, 1.7±1.2 h in kidneys, 2.4±1.9 h in liver, 1.5±0.3 h in urinary bladder and 9.4±5.5 h in the remainder of the body; the mean residence time in tumour was 0.47 h (range: 0.03–6.50 h). Based on our findings, the predicted absorbed doses for 90Y-DOTATOC would be 7.6±6.3 (spleen), 3.3±2.2 (kidneys), 0.7±0.6 (liver), 2.2±0.3 (bladder), 0.03±0.01 (red marrow) and 10.1 (range: 1.4–31.0) (tumour) mGy/MBq. These results indicate that high activities of 90Y-DOTATOC can be administered with low risk of myelotoxicity, although with potentially high radiation doses to the spleen and kidneys. Tumour doses were high enough in most cases to make it likely that the disired therapeutic response desired would be obtained.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 17 February and in revised form 22 April 1999
Rights and permissions
About this article
Cite this article
Cremonesi, M., Ferrari, M., Zoboli, S. et al. Biokinetics and dosimetry in patients administered with 111In-DOTA-Tyr3-octreotide: implications for internal radiotherapy with 90Y-DOTATOC. Eur J Nucl Med 26, 877–886 (1999). https://doi.org/10.1007/s002590050462
Issue Date:
DOI: https://doi.org/10.1007/s002590050462