Abstract
Purpose
A [11C]UCB-J blocking study was performed in healthy volunteers to validate simplified, non-invasive measures for quantifying presynaptic SV2A expression using subcortical white matter as reference tissue.
Methods
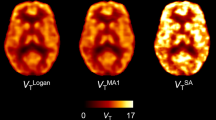
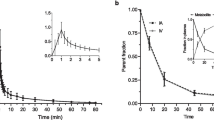
Ninety minutes dynamic [11C]UCB-J PET scanning with arterial blood sampling was performed in 10 healthy volunteers (8 M/2F; age 27.6 ± 10.0 yrs), before and after administration of a novel chemical entity with selective affinity for SV2A. The centrum semi-ovale (SO) was validated as reference region by comparing baseline and post treatment distribution volume (VT). Using SO as reference tissue, Binding Potential (BPSO) using a Simplified Reference Tissue Model (SRTM, down to 60 min acquisition) and Standardized Uptake Value Ratios (60-90 min post injection - SUVRSO,60-90min) were compared with regional distribution volume ratios (DVR). Next, SV2A occupancy values based on SRTM BPSO and SUVRSO,60-90min were compared to occupancy estimates using regional VT values and a Lassen plot.
Results
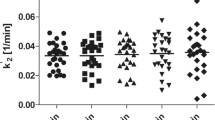
After pretreatment, regional VT values were reduced significantly except for SO. Highly significant correlations were found between DVR, SRTM BPSO and SUVRSO,60–90min. Compared to DVR, baseline SRTM BPSO showed a small bias (≤ 6.1%) with lower precision for shorter acquisition times, while SUVRSO,60-90min showed 3.5% bias with similar precision. Differences between SV2A occupancy values based on SUVRSO,60-90min and occupancy estimates using VT and a Lassen plot were small but significant, while negligible bias was found for SRTM based occupancy estimates (at least 70 min acquisition).
Conclusion
This [11C]UCB-J blocking study validated SO as a suitable reference region for non-invasive quantification of SV2A availability and drug occupancy in the human brain. Accurate quantification can be achieved by using either SUVRSO,60-90min with a 60–90 min PET acquisition or SRTM BPSOwith at least 70 min dynamic PET acquisition.





Similar content being viewed by others
References
Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–35.
Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348ra96. https://doi.org/10.1126/scitranslmed.aaf6667.
Nabulsi NB, Mercier J, Holden D, Carre S, Najafzadeh S, Vandergeten MC, et al. Synthesis and preclinical evaluation of [11C]UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med. 2016;57:777–84. https://doi.org/10.2967/jnumed.115.168179.
Crevecoeur J, Kaminski RM, Rogister B, Foerch P, Vandenplas C, Neveux M, et al. Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol Appl Neurobiol. 2014;40:191–204. https://doi.org/10.1111/nan.12054.
Feng G, Xiao F, Lu Y, Huang Z, Yuan J, Xiao Z, et al. Down-regulation synaptic vesicle protein 2A in the anterior temporal neocortex of patients with intractable epilepsy. J Mol Neurosci. 2009;39:354–9. https://doi.org/10.1007/s12031-009-9288-2.
Proper EA, Oestreicher AB, Jansen GH, Veelen CW, van Rijen PC, Gispen WH, et al. Immunohistochemical characterization of mossy fibre sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain J Neurol. 2000;123(Pt 1):19–30.
DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. https://doi.org/10.1002/ana.410270502.
Hamos JE, DeGennaro LJ, Drachman DA. Synaptic loss in Alzheimer’s disease and other dementias. Neurology. 1989;39:355–61.
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–43. https://doi.org/10.1016/j.neuron.2014.07.040.
Kang H, Voleti B, Hajszan T, Rajkowska G, Stockmeier C, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7. https://doi.org/10.1038/nm.2886.
Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. https://doi.org/10.1038/nature16549.
Lubberink M, Appel L, Daging J, Lindskog K, Danfors T, Larsson E-M, et al. Tracer kinetic analysis of the SV2A ligand [11C]UCB-a as a PET marker for synaptic density in humans. J Nucl Med. 2017;58:631.
Bretin F, Bahri MA, Bernard C, Warnock G, Aerts J, Mestdagh N, et al. Biodistribution and radiation dosimetry for the novel SV2A radiotracer [18F]UCB-H: first-in-human study. Mol Imaging Biol. 2015;17:557–64. https://doi.org/10.1007/s11307-014-0820-6.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40. https://doi.org/10.1097/00004647-199609000-00008.
Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab. 2010;30:46–50. https://doi.org/10.1038/jcbfm.2009.190.
Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–52. https://doi.org/10.1097/01.wcb.0000033967.83623.34.
Finnema SJ, Nabulsi NB, Mercier J, Lin SF, Chen MK, Matuskey D, et al. Kinetic evaluation and test-retest reproducibility of [11C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2017:271678x17724947. https://doi.org/10.1177/0271678x17724947.
Kletting P, Glatting G. Model selection for time-activity curves: the corrected Akaike information criterion and the F-test. Z Med Phys. 2009;19:200–6.
Glatting G, Kletting P, Reske SN, Hohl K, Ring C. Choosing the optimal fit function: comparison of the Akaike information criterion and the F-test. Med Phys. 2007;34:4285–92. https://doi.org/10.1118/1.2794176.
Golla SSV, Adriaanse SM, Yaqub M, Windhorst AD, Lammertsma AA, van Berckel BNM, et al. Model selection criteria for dynamic brain PET studies. EJNMMI Phys. 2017;4:30. https://doi.org/10.1186/s40658-017-0197-0.
Alves IL, Vallez Garcia D, Parente A, Doorduin J, Dierckx R, Marques da Silva AM, et al. Pharmacokinetic modeling of [11C]flumazenil kinetics in the rat brain. EJNMMI Res. 2017;7:17. https://doi.org/10.1186/s13550-017-0265-4.
Slifstein M, Laruelle M. Effects of statistical noise on graphic analysis of PET neuroreceptor studies. J Nucl Med. 2000;41:2083–8.
Wong K-P, Wardak M, Shao W, Dahlbom M, Kepe V, Liu J, et al. Quantitative analysis of [18F]FDDNP PET using subcortical white matter as reference region. Eur J Nucl Med Mol Imaging. 2010;37:575–88. https://doi.org/10.1007/s00259-009-1293-8.
Van Laere K, Ahmad RU, Hudyana H, Dubois K, Schmidt ME, Celen S, et al. Quantification of [18F]JNJ-42259152, a novel phosphodiesterase 10A PET tracer: kinetic modeling and test-retest study in human brain. J Nucl Med. 2013;54:1285–93. https://doi.org/10.2967/jnumed.112.118679.
Acknowledgments
We thank Kwinten Porters and Jef Van Loock for their technical assistance, and the radiopharmacy team for the tracer productions. Part of this study was sponsored by a research grant of UCB to KU Leuven (principal investigator Koen Van Laere).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Brigitte Lacroix, Joel Mercier, David Sciberras and Paul Maguire are employees of UCB Pharma. Michel Koole, June van Aalst, Martijn Devrome, Nathalie Mertens, Kim Serdons and Koen Van Laere have no conflicts to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
ESM 1
(DOCX 17581 kb)
Rights and permissions
About this article
Cite this article
Koole, M., van Aalst, J., Devrome, M. et al. Quantifying SV2A density and drug occupancy in the human brain using [11C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur J Nucl Med Mol Imaging 46, 396–406 (2019). https://doi.org/10.1007/s00259-018-4119-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4119-8