Abstract
Purpose
Pheochromocytomas/paragangliomas (PHEOs/PGLs) overexpress somatostatin receptors and recent studies have already shown excellent results in the localization of these tumors using 68Ga-labeled somatostatin analogs (68Ga-DOTA-SSA), especially in patients with germline succinate dehydrogenase subunit B gene (SDHB) mutations and head and neck PGLs (HNPGLs). The value of 68Ga-DOTA-SSA has to be established in sporadic cases, including PHEOs. Thus, the aim of this study was to compare 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT, and conventional imaging in patients with various PHEOs/PGLs with a special emphasis on sporadic cases, including those located in the adrenal gland.
Design
68Ga-DOTATATE, 18F-FDOPA PET/CT, and conventional imaging (contrast-enhanced CT and MRI with MR angiography sequences) were prospectively performed in 30 patients (8 with SDHD mutations, 1 with a MAX mutation and 21 sporadic cases) with PHEO/PGL at initial diagnosis or relapse.
Results
The patient-based sensitivities were 93 % (28/30), 97 % (29/30), and 93 % (28/30) for 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT, and conventional imaging, respectively. The lesion-based sensitivities were 93 % (43/46), 89 % (41/46), and 76 % (35/46) for 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT, and conventional imaging respectively (p = 0.042). 68Ga-DOTATATE PET/CT detected a higher number of HNPGLs (30/30) than 18F-FDOPA PET/CT (26/30; p = 0.112) and conventional imaging (24/30; p = 0.024). 68Ga-DOTATATE PET/CT missed two PHEOs of a few millimeters in size and a large recurrent PHEO. One lesion was considered false-positive on 68Ga-DOTATATE PET/CT and corresponded to a typical focal lesion of fibrous dysplasia on MRI. Among the 11 lesions missed by conventional imaging, 7 were detected by conventional imaging with knowledge of the PET results (4 HNPGLs, 2 LNs, and 1 recurrent PHEO).
Conclusion
68Ga-DOTATATE PET/CT is the most sensitive tool in the detection of HNPGLs, especially SDHD-related tumors, which may be very small and fail to concentrate sufficient 18F-FDOPA. The present study further expands the use of 68Ga-DOTATATE for all patients with HNPGLs, regardless of their genotype. 68Ga-DOTATATE PET/CT may be inferior to 18F-FDOPA PET/CT in the detection PHEOs.
Similar content being viewed by others
Introduction
Paragangliomas (PGLs) arise from head/neck and thoracic parasympathetic paraganglia, or extra-adrenal clusters of chromaffin cells that persist postnatally, and are aligned along thoracoabdominal sympathetic chains. Those arising from the adrenal medulla are called pheochromocytomas (PHEOs). These tumors are often characterized by recurrent behavior, multiplicity, and metastases – especially since more than 30 % of these are hereditary, and another 15 – 20 % carry somatic mutations in one of more than a dozen well-characterized PHEO/PGL susceptibility genes. Therefore, the role of whole-body (WB) PHEO/PGL-specific functional imaging is rapidly increasing as a part of precision medicine in these patients, together with their diagnostic and therapeutic planning [1, 2].
Nowadays, WB PHEO/PGL-specific functional imaging may accurately confirm or rule out PHEO/PGL, define its extension, determine its multiplicity, and finally, exclude metastasis [3]. To this end, nuclear imaging must still be complemented by anatomical imaging, including CT and MR imaging – both of which are considered conventional imaging. Nevertheless, the major advantage of nuclear imaging is to provide a high visual contrast between tumor and healthy tissue, which enables the detection of tumors that could potentially be missed by conventional imaging. However, CT provides critical information for the assessment of tumor extension into bone and surrounding soft tissues. MR angiography (MRA) sequences have also shown excellent sensitivities and specificities [4–6]. There is a recent trend for using 3D time-of-flight (TOF) imaging and time-resolved 4D gadolinium MRA for tumor characterization in the head and neck area.
Over the last 15 years 18F-FDOPA PET/CT has been found to be the most sensitive imaging modality for these tumors, especially in the head and neck area. More recently, PET/CT imaging with 68Ga-labeled somatostatin analogs (68Ga-DOTA-SSAs) has rapidly evolved, since it does not require a cyclotron to make the radiotracer. Moreover, a special advantage of labeled SSAs is that, unlike 18F-FDOPA, they can be used in the radioactive treatment of these tumors (as theranostic agents). In 2014, 68Ga-DOTATATE was approved as an orphan drug by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the imaging of gastroenteropancreatic neuroendocrine tumors. The use 68Ga-DOTA-SSA in the context of PHEO/PGLs has been less studied, but has shown excellent preliminary results in localizing these tumors [7–12]. 68Ga-DOTA-SSA and 18F-FDOPA PET have been compared in only three studies: one retrospective study from Innsbruck Medical University (68Ga-DOTATOC in 20 patients with unknown genetic background) and two prospective studies from the NIH (68Ga-DOTATATE in 17 and 20 patients). In these studies, 68Ga-DOTA-SSAs PET/CT detected more primary head and neck PGLs (HNPGLs) as well as metastatic succinate dehydrogenase subunit B-related PGLs than 18F-FDOPA PET/CT [13–15]; however, none of these studies addressed the usefulness of 68Ga-DOTATATE in sporadic cases including PHEOs.
Thus, the aim of this prospective study was to extend recent data related to 68Ga-DOTATATE and 18F-FDOPA PET/CT in these tumors by comparing 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT and conventional imaging while also including MRA for HNPGLs in a series of 30 patients with PHEOs/PGLs of different genetic backgrounds and localizations, including PHEOs in which the role of 68Ga-DOTA-SSA has not yet been established.
Materials and methods
Eligibility criteria
The inclusion criteria were: age ≥18 years, PHEO or PGL at initial staging or restaging, and reference imaging within the last 2 months including a multiphasic cervicothoracoabdominopelvic CT scan, 18F-FDOPA PET/CT and head and neck MR imaging (in patients with HNPGL). Pregnant or breastfeeding women were excluded. All patients gave signed informed consent to participation. The study was approved by the local ethics committee (Comité de Protection des Personnes Sud-Méditerranée II) and the French drug and device Regulation Agency (ANSM). The study has been registered at ClinicalTrials.gov (identifier: NCT02186678).
Study design
The present study was a prospective, open-label single-center study. All patients were evaluated by 18F-FDOPA PET/CT, multiphasic cervicothoracoabdominopelvic CT scan, head and neck MRI (in patients with HNPGL), and 68Ga-DOTATATE PET/CT. During the study period, PET/CT and conventional imaging examinations were interpreted in standard clinical fashion with knowledge of the patients' clinical context and available imaging studies. At the end of the study, all examinations were interpreted in a blinded fashion by two experienced nuclear physicians and radiologists.
68Ga-DOTATATE preparation
The whole 68Ga-DOTATATE preparation process was automated through the EluSynthGa68 synthesis module (Iason, Graz-Seiersberg, Austria) placed in a shielded hot cell under class-A/ISO4.8 conditions in our radiopharmacy unit. The DOTATATE lyophilisate (Iason) is dissolved in water for injection and 1.14 M acetate buffer is added to the peptide solution. Prior to synthesis, this solution is transferred into the module reactor as a receiver. 68Ga-chloride ([68GaCl3) is obtained through the elution of a 68Ga/68Ge generator (Iason). The resulting 68Ga-chloride solution is prepurified onto a preconditioned SCX cartridge (Bond-Elut SCX, Agilent) and eluted into the reaction vial with the DOTA peptide. The labelling reaction occurs at 95 °C, pH 4.5, for 8 min. After labelling, the raw product is loaded onto a RP tC18 cartridge (Wat03605, Waters). The product is washed with water for injection (5 mL twice).
Desorption of the product from the reversed-phase cartridge is achieved by washing with 2 mL aqueous 30 % ethanol solution into the collection vial, which contains 10 mL NaCl 0.9 % for injection. This solution is then sterilized by filtration through a 0.22-μm membrane filter (Millex-GV 33 mm, PVDF 0.22 μm; EMD Millipore) and collected in a sterile product vial. The final product is a clear and sterile solution. Quality controls include activity measurements, physical half-life measurement, bubble test of the sterilizing filter, radiochemical purity assessed by HPLC in a water/acetonitrile gradient, and radionuclide purity by γ-radioactivity TLC scanner. The solution was also checked for endotoxins and microbiologic safety.
18F-FDOPA preparation
6-18F-Fluoro-l-3,4-dihydroxyphénylalanine (Iasodopa®; Iason) was shipped in an acetic acid solution (1.05 mg/mL, pH 2.3 – 3.0) for reasons of radiochemical stability. Before administration, the pH was adjusted to 4.0 – 5.0 by adding a sterile sodium bicarbonate solution (8.4 g/100 mL, used at 100 μL/mL IASOdopa). The preparation can be administered up to 2 h after the pH adjustment to avoid further product oxidation according to the manufacturer’s instructions.
PET/CT acquisition protocols and reconstruction parameters
Images were acquired using a Siemens 3D tomograph (Biograph 16 TruePoint). A low-dose CT scan was performed for both PET/CT protocols with the following parameters: 110 kV, 100 mA, 24.7 s, and pitch 1. PET data were reconstructed with a 6-mm FWHM filter in a pixel matrix (168 × 168 for the WB acquisition/256 × 256 for the craniocervical scan) using 3D OSEM with eight subsets and four iterations. Correction attenuation was performed with the CT scan and Gaussian filter smoothing (6 mm for the WB acquisition/ 4 mm for the craniocervical scan). For 18F-FDOPA PET/CT, all patients fasted for at least 3 h. Image acquisition was started 45 min (20 – 60 min, median 47.5 min) after injection of 3.5 MBq/kg of IASOdopa® (1.8 – 5 MBq/kg, median 3.4 MBq/kg). For 68Ga-DOTATATE, all patients fasted for at least 3 h. Image acquisition was started 45 min (40 – 80 min, median 45 min) after injection of 2 MBq/kg of 68Ga-DOTATATE (1.4 – 2.9 MBq/kg, median 2.3 MBq/kg). The acquisition protocol was started with a craniocervical scan (one bed position of 10 minutes, headboard, arms along the body), followed by a WB acquisition from the top of the head to the mid-thigh (3 min per bed position, arms above the head).
Image analysis and quantification of PET/CT examinations
CT, PET (corrected), and fusion images were displayed for visual analysis on a syngo.via workstation with image fusion software (Siemens Medical Solutions, Knoxville, TN). PET/CT scans were interpreted by experienced nuclear medicine physicians. Each focus of increased extra physiologic radiotracer uptake was recorded and interpreted according to the particular case. Quantitative analysis was performed on positive foci on visual analysis and included the standardized uptake values (SUVmax, SUVmean) which were obtained from volumes of interest and the metabolic tumor volume (MTV) which was calculated for a threshold of 42 % of the SUVmax (also referred to as MTV 42 %).
CT imaging
Multiphasic contrast-enhanced thoracoabdominopelvic CT scans were performed with a 64-section multidetector CT scanner (LightSpeed Discovery CT750 HD; GE Medical Systems, Milwaukee, WI) using the following standardized protocol. Patients were positioned supine head first in the scanner. An 18-gauge cannula was placed in the right or left antecubital vein. The scanning protocol consisted of three helical scans obtained in an automated, predetermined, and timed sequence. Scanning parameters were 120 kVp, 180 – 375 mA (Auto mA; GE Medical Systems), rotation time 0.7 s, pitch 1.375 and 0.625-mm detector configuration, with beam width 40 mm. Precontrast abdominopelvic CT scans were performed prior to administration of contrast medium. A region of interest (ROI) was drawn on the descending aorta to avoid artifacts caused by high concentrations of contrast material. Iodine (350 mg/mL, 2 ml/kg; Xenetix; Guerbet, Roissy, France) was injected at a rate of 4 mL/s using an automatic injector (Injektron CT2 CT; Medtron, Saarbrücken, Germany). Using a bolus tracking technique, the arterial thoracoabdominal CT scan was obtained with a scan delay of 5 s after a threshold trigger of 100 HU on the ROI was reached. The portal abdominopelvic CT scan was performed with a scan delay of 40 s after completion of the arterial phase.
MR imaging
MR imaging was performed on a 3 T MR scanner (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) equipped with an 32-channel phased-array head coil. The conventional MR imaging protocol included the following sequences: T1-W imaging (TR 500 ms, TE 8.5 ms, section thickness 3 mm, FOV 190 mm), T2-W imaging (TR 6,050 ms, TE 109 ms, section thickness 4 mm, FOV 220 mm), 3D TOF (TR 21 ms, TE 3.43 ms, section thickness 0.6 mm, flip angle 18°, FOV 193 × 219 mm), contrast-enhanced time-resolved 4D-MRA (TWIST, TR 2.51 ms, TE 0.98 ms, section thickness 1 mm, flip angle 17°, FOV 288 × 234 mm, phase duration time 2.7 s), 3D T1-W after contrast agent administration with fat saturation (TR 5.94 ms, TE 2.46 ms, section thickness 0.9 mm, flip angle 18°, FOV 220 mm).
Gold standard
Histology was considered the gold standard for the diagnosis of PHEO/PGL. In cases where no surgical resection was performed, the diagnosis of PGL was made by the confrontation of the different imaging modalities and by reaching a consensus between experienced radiologists and nuclear physicians.
Statistical analysis
Sensitivities were calculated for patients and for individual lesions. The differences in sensitivity between the three imaging procedures were compared using the chi-squared test and Fisher's exact test when n was <5. SUVmax, SUVmean and MTV 42 % were compared between 68Ga-DOTATATE PET/CT and 18F-FDOPA PET/CT using a Wilcoxon signed ranks test. Statistical analysis was performed using IBM SPSS Statistics version 20 (IBM SPSS Inc., Chicago, IL). For all tests, a two-sided p value <0.05 was considered statistically significant.
Results
Patient and tumor characteristics
Included in the study were 30 patients (23 women, 7 men, age range 22 to 84 years) who fulfilled the inclusion criteria. Nine patients (30 %) harbored germline mutations, specifically SDHD (eight patients) and MAX (MYC-associated factor X; one patient). The total number of PGL lesions was 46 based on the total number of lesions depicted by all imaging studies. Lesions were distributed as follows: 30 HNPGLs (18 SDHD, 12 sporadic), 11 PHEOs (7 primary and 4 recurrent, all but one sporadic) and 5 metastases from two sporadic cases (Supplementary Table 1). One patient with a previous history of bilateral adrenalectomy for MAX-related PHEOs had no recurrent tumor on conventional imaging and PET studies despite symptoms of relapse and elevated urinary and plasma normetanephrine (four times the upper reference limit). The diagnosis was confirmed histologically at 23 of 46 sites (50 %). Seven patients harbored more than one lesion.
Patient-based and lesion-based sensitivities
The patient-based sensitivities were 93 % (28/30, 95 % CI 78.7 – 98.2 %), 97 % (29/30, 95 % CI 83.3 – 99.4 %) and 93 % (28/30, 95 % CI 78.7 – 98.2 %) for 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT and conventional imaging, respectively. The lesion-based sensitivities (blinded analysis) were 93 % (43/46, 95 % CI 82.5 – 97.8 %), 89 % (41/46, 95 % CI 76.9 – 95.3 %) and 76 % (35/46, 95 % CI 76.9 – 95.3 %) for 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT and conventional imaging, respectively (p = 0.042). Tumor 68Ga-DOTATATE SUVmax, SUVmean and MTV were higher than those observed with 18F-FDOPA (p < 0.001; Supplementary Table 1).
68Ga-DOTATATE PET/CT vs. 18F-FDOPA PET/CT in HNPGLs
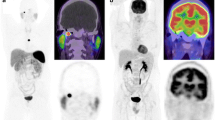
68Ga-DOTATATE PET/CT detected all 30 lesions in the head and neck area, 18F-FDOPA PET/CT detected 26 lesions (86.7 %, 95 % CI 70.3 – 94.7 %; p = 0.112) and MR imaging detected 24 lesions (80 %, 95 % CI 62.7 – 90.5 %; p = 0.024; Table 1). 18F-FDOPA PET/CT missed four SDHD-related HNPGLs (Supplementary Table 1). All but one of these lesions were and a few millimeters in size. In a patient with multiple SDHD-associated PGLs, a 37-mm vagal PGL failed to concentrate 18F-FDOPA (patient 17, Fig. 1). In one patient, WB acquisition was inferior to craniocervical acquisition, and failed to detect a small carotid body PGL (patient 29, Fig. 2).
Multicentric SDHD-related PGL syndrome. a 68Ga-DOTATATE maximal intensity projection (MIP) image shows three PGLs (right vagal, left vagal, left jugular). b 18F-FDOPA PET MIP image fails to show the large left vagal PGL (arrow). c Contrast-enhanced time-resolved 4D MRA images at 3 T shows a small nodule located in the wall of the jugular bulb with early arterial enhancement (arrows), prior to filling of the jugular vein and sigmoid sinus lumen with contrast medium (asterisks). This lesion was detected only upon knowledge of the PET results
Multicentric SDHD-related PGL syndrome. a Craniocervical 68Ga-DOTATATE MIP image shows two vagal PGLs. b. Craniocervical 18F-FDOPA PET MIP image shows the two lesions but was slightly positive on the left side (arrow). c WB 68Ga-DOTATATE MIP image shows the two known lesions. d WB 18F-FDOPA PET MIP image fails to show the left vagal PGL. e Early arterial phase contrast-enhanced time-resolved 4D MRA image at 3 T. f 4D MRA image fused with 3D gadolinium-enhanced T1-W fat-saturated (3D VIBE) image. Both images show the early arterial enhancement pattern of the two vagal PGLs (white arrows left vagal PGL). Note the discrete splaying between the internal carotid artery (yellow arrowheads) and the external carotid artery (green arrowheads)
68Ga-DOTATATE PET/CT vs. 18F-FDOPA PET/CT in PHEOs
Among the seven patients who were found to have PHEO at initial diagnosis, 68Ga-DOTATATE PET/CT was positive in six with highly elevated SUVs (Supplementary Table 1). 68Ga-DOTATATE PET/CT failed to identify one PHEO that was a small SDHD-related PHEO located within a highly avid normal gland. 18F-FDOPA PET/CT was positive in all seven patients. Histologic confirmation was obtained in six of the seven patients. The diagnosis in one patient was made by the multidisciplinary team.
Among the three patients with recurrent sporadic PHEO, 68Ga-DOTATATE PET/CT detected fewer lesions than 18F-FDOPA PET/CT (two vs. four of four lesions, p = 0.429). One lesion was a few millimeters in size and was located close to the surgical clips (patient 10). The second lesion was a large recurrent PHEO that was considered negative due to the very low tumor uptake (patient 13, Fig. 3).
Sporadic recurrent PHEO. a Multiphasic CT scan shows a left retroperitoneal mass separated from the mid-kidney fascia (arrows) that shows early arterial phase enhancement. b Fused axial 68Ga-DOTATATE PET/CT image centered over the tumor. The lesion shows lower 68Ga-DOTATATE uptake (long arrow) than the normal contralateral adrenal (short arrow) and was considered negative. c Fused axial 18F-FDOPA PET/CT image centered over the tumor showing a highly 18F-FDOPA-avid lesion (arrow)
False-positive lesion
In a single patient with PGL, one focus was considered as false-positive on 68Ga-DOTATATE PET/CT and corresponded to a typical focal lesion of fibrous dysplasia on MR and CT imaging (patient 3, Fig. 4). This lesion was 18F-FDOPA-negative.
Coexistence of jugular PGL and focal fibrous dysplasia. a 18F-FDOPA PET MIP image shows a right jugular PGL. b 68Ga-DOTATATE MIP image shows a left jugular PGL and an additional contralateral focus. c Fused axial 68Ga-DOTATATE PET/CT image centered over the lesions. d CT scan shows a cystic bone lesion in the temporal marrow space next to the petroclival suture. e 3D TOF sequence image shows the absence of arterial feeding branches. Note the typical high-velocity arterial branches within the jugular PGL. f T2-W image shows high signal
Blinded vs. open analysis
In the blinded analysis, 11 lesions were missed by conventional imaging, including three jugular PGLs, two vagal PGLs, one PGL of the recurrent laryngeal nerve, three PHEOs (two recurrent and one at initial staging), and two lymph node lesions. All these lesions were only a few millimeters in size. Seven of these lesions were detected by conventional imaging with knowledge of the PET results: four of seven HNPGLs (three of three jugular PGL and one of two vagal PGLs) and two of two cervical lymph node lesions by 4D MRA and 3D TOF imaging and one recurrent PHEO nodule detected by CT. In the analysis of each PET study, no differences was observed when knowledge of the results of contrast-enhanced CT an MR imaging were known.
Discussion
To the best of our knowledge, this is the largest prospective study that evaluated the sensitivity of 68Ga-DOTATATE PET/CT in comparison with that of 18F-FDOPA PET/CT and conventional imaging (including MRA sequences). The principal conclusions that can be drawn from this study include:
-
1.
A higher sensitivity of 68Ga-DOTATATE PET/CT than 18F-FDOPA PET/CT for HNPGLs
-
2.
The presence of 18F-FDOPA-negative HNPGLs in SDHx mutation carriers
-
3.
A high sensitivity of 68Ga-DOTATATE PET/CT in the detection of PHEOs despite a lower number of lesions possibly depicted by this modality than by 18F-FDOPA PET/CT
-
4.
An improvement in detection rates of tumors by conventional imaging interpreted alongside the results of PET imaging
Until recently, 18F-FDOPA PET/CT was considered as the most sensitive imaging modality for the detection of HNPGLs [16–18]. The results of the present study confirm the recent results reported by Kroiss et al. [13] and Janssen et al. [15], and support the use of PET/CT with 68Ga-DOTATATE and perhaps other 68Ga-labeled DOTA SSAs as the preferred functional imaging modality for HNPGLs. The high sensitivity of 68Ga-DOTATATE PET/CT can be largely attributed to the high tumor-to-background uptake ratio. 18F-FDOPA also has a favorable biodistribution for the detection of HNPGLs, but the concentration of this tracer in these tumors is lower than that of 68Ga-DOTATATE. It could be argued that the detection of small PGLs would have a limited impact in the management of patients. However, surgery for HNPGLs may lead to cranial nerve palsy in a high number of patients that may significantly compromise additional surgery (especially in the contralateral side). Therefore, identification of additional small tumors may change the management strategy from surgery to radiosurgery or observation. Further prospective studies are needed to answer this important question.
It is well known that SST-based imaging may be somewhat less specific than 18F-FDOPA PET imaging in the evaluation of these tumors and could be falsely positive, mainly in metastatic lymph nodes due to various cancers, meningiomas, and inflammatory processes [12]. Usually, this is not a serious issue, since HNPGLs have specific locations and exhibit high uptake values. In the present study, we present a patient with a temporal bone fibrous dysplasia close to the jugular foramen mimicking a jugular PGL. Fibrous dysplasia of bone (e.g., McCune-Albright syndrome, oncogenic osteomalacia) has been shown to concentrate radiolabeled SSA [12, 19–21], and therefore may represent a potential pitfall for 68Ga-DOTA-SSA imaging.
Some of our patients had false-negative results on 18F-FDOPA PET/CT. All of these patients were SDHD mutation carriers. Such false-negative findings have been mainly reported in patients with PGLs of sympathetic origin or small HNPGLs [18, 22, 23]. One of our patients had a large vagal PGL that failed to concentrate the tracer. The molecular genetics of this metabotype are currently unexplained, but epigenetic mechanisms (histones and methylation modifications) might be involved in addition to genetic mutations [24]. The evaluation of latency-associated transcript expression in different PGLs in the same patient would be of particular interest for explaining such phenotypical heterogeneity.
We also found a high sensitivity of 68Ga-DOTATATE PET/CT in the evaluation of PHEOs, better than that expected with octreoscan, and is in agreement with the findings of in vitro studies showing a high proportion of tumors with SST2 overexpression (>80 %) [25]. One of the main drawbacks of 68Ga-DOTA-SSA is the very high physiologic uptake by healthy adrenal glands [26]. This could be a critical problem in the detection of small PHEOs in hereditary syndromes, mainly related to multiple endocrine neoplasia type 2 (MEN2) and neurofibromatosis type 1 (NF1) but also to Von Hippel-Lindau (VHL) or PGL syndromes associated with mutations in one of the succinate dehydrogenase subunits. It should be noted that in our study, a small PHEO was not detected by 68Ga-DOTA-SSA in a patient with SDHD mutation. This is a clear advantage of 18F-FDOPA PET/CT, due to the absence or very low physiologic uptake by the adrenals. 68Ga-DOTA-SSA uptake is probably due to adrenal cortex activity rather than medullary activity and should therefore possibly be inhibited by proper premedication, but this remains to be evaluated by preclinical or clinical studies [27]. However, we also showed that this limitation was not only due to the to high uptake of 68Ga-DOTATATE in the adrenals (with low tumor-to-organ uptake) but also to possible low tumor uptake. It would be important to compare SST receptor expression in patients with sporadic PHEO in comparison with that in patients with different types of hereditary PHEO.
Finally, 68Ga-DOTATATE PET/CT surpassed the combination of classical MRI sequences and MRA in the detection of HNPGLs. In some patients, MRI was not able reliably to distinguish between PGLs and other tumors. However, MRI remains necessary in combination with PET imaging, as it allows precise delineation of tumor extent and provides critical information for tumor characterization [3]. We clearly showed that the detection rate of MRI is improved by its interpretation alongside PET findings and that 4D MRA and TOF sequences are reliable tools for detecting very small jugular and vagal PGLs. TOF MRA is robust and easy to implement in other sequences but is extremely time consuming. Therefore, in our study, we limited the field of exploration to the skull base. We recommend personalizing the MR acquisition protocol according to the PET findings with the following sequences: T1-W/T2-W imaging, contrast-enhanced time-resolved 4D MRA, 3D T1-W imaging after contrast agent administration with fat saturation, 3D TOF acquisition centered on positive PET foci and eventually MR spectroscopy for larger tumors [28]. Combining PET and MRI in integrated systems would be a very interesting approach for localizing and characterizing these tumors [29]. The present study also provided the opportunity to show exceptional images of jugular PGLs of the order of millimeters in size, and illustrated their origin within the pars vascularis compartment of the jugular foramen within the wall of the jugular bulb. Radiologists should be aware of this precise location in order to facilitate their detection.
We acknowledge several limitations to our study: small series, and various hereditary tumors (sympathetic extraadrenal PGLs, VHL, MEN2 and NF1) were not included. Histologic proof was not available for all tumors, but this is acceptable in patients in whom biopsies or surgical intervention would be very difficult, dangerous, and even unethical [30].
In conclusion, 68Ga-DOTATATE PET/CT is a very sensitive tool in the detection of HNPGLs, especially SDHD mutation-related tumors which may be very small and fail to concentrate sufficient 18F-FDOPA. The present study further expands the use of 68GA-DOTATE for all patients with HNPGLs, regardless of the genotype. 68Ga-DOTATATE PET/CT may be inferior to 18F-FDOPA PET/CT in the detection PHEOs.
References
Castinetti F, Kroiss A, Kumar R, Pacak K, Taieb D. 15 years of paraganglioma: imaging and imaging-based treatment of pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2015;22:T135–45. doi:10.1530/ERC-15-0175.
Taieb D, Hicks RJ, Pacak K. Radiopharmaceuticals in paraganglioma imaging: too many members on board? Eur J Nucl Med Mol Imaging. 2015. doi:10.1007/s00259-015-3213-4.
Taieb D, Kaliski A, Boedeker CC, Martucci V, Fojo T, Adler Jr JR, et al. Current approaches and recent developments in the management of head and neck paragangliomas. Endocr Rev. 2014;35:795–819. doi:10.1210/er.2014-1026.
van den Berg R, Schepers A, de Bruine FT, Liauw L, Mertens BJ, van der Mey AG, et al. The value of MR angiography techniques in the detection of head and neck paragangliomas. Eur J Radiol. 2004;52:240–5. doi:10.1016/j.ejrad.2003.12.002.
Neves F, Huwart L, Jourdan G, Reizine D, Herman P, Vicaut E, et al. Head and neck paragangliomas: value of contrast-enhanced 3D MR angiography. AJNR Am J Neuroradiol. 2008;29:883–9. doi:10.3174/ajnr.A0948.
Gravel G, Niccoli P, Rohmer V, Moulin G, Borson-Chazot F, Rousset P, et al. The value of a rapid contrast-enhanced angio-MRI protocol in the detection of head and neck paragangliomas in SDHx mutations carriers: a retrospective study on behalf of the PGL.EVA investigators. Eur Radiol. 2015. doi:10.1007/s00330-015-4024-5.
Naji M, Al-Nahhas A. (68)Ga-labelled peptides in the management of neuroectodermal tumours. Eur J Nucl Med Mol Imaging. 2012;39 Suppl 1:61–7. doi:10.1007/s00259-011-1990-y.
Naji M, Zhao C, Welsh SJ, Meades R, Win Z, Ferrarese A, et al. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2011;13:769–75. doi:10.1007/s11307-010-0396-8.
Sharma P, Thakar A, Suman Kc S, Dhull VS, Singh H, Naswa N, et al. 68Ga-DOTANOC PET/CT for baseline evaluation of patients with head and neck paraganglioma. J Nucl Med. 2013;54:841–7. doi:10.2967/jnumed.112.115485.
Kroiss A, Shulkin BL, Uprimny C, Frech A, Gasser RW, Url C, et al. (68)Ga-DOTATOC PET/CT provides accurate tumour extent in patients with extraadrenal paraganglioma compared to (123)I-MIBG SPECT/CT. Eur J Nucl Med Mol Imaging. 2015;42:33–41. doi:10.1007/s00259-014-2892-6.
Sharma P, Mukherjee A, Karunanithi S, Naswa N, Kumar R, Ammini AC, et al. Accuracy of 68Ga DOTANOC PET/CT imaging in patients with multiple endocrine neoplasia syndromes. Clin Nucl Med. 2015;40:e351–6. doi:10.1097/RLU.0000000000000775.
Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500–16. doi:10.1148/rg.352140164.
Kroiss A, Putzer D, Frech A, Decristoforo C, Uprimny C, Gasser RW, et al. A retrospective comparison between (68)Ga-DOTA-TOC PET/CT and (18)F-DOPA PET/CT in patients with extra-adrenal paraganglioma. Eur J Nucl Med Mol Imaging. 2013;40:1800–8. doi:10.1007/s00259-013-2548-y.
Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, et al. Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2015;21:3888–95. doi:10.1158/1078-0432.CCR-14-2751.
Janssen I, Chen CC, Taieb D, Patronas NJ, Millo CM, Adams KT, et al. 68Ga-DOTATATE PET/CT in the localization of head and neck paragangliomas compared to other functional imaging modalities and CT/MRI. J Nucl Med. 2015. doi:10.2967/jnumed.115.161018.
Charrier N, Deveze A, Fakhry N, Sebag F, Morange I, Gaborit B, et al. Comparison of [111In]pentetreotide-SPECT and [18F]FDOPA-PET in the localization of extra-adrenal paragangliomas: the case for a patient-tailored use of nuclear imaging modalities. Clin Endocrinol. 2011;74:21–9. doi:10.1111/j.1365-2265.2010.03893.x.
King KS, Chen CC, Alexopoulos DK, Whatley MA, Reynolds JC, Patronas N, et al. Functional imaging of SDHx-related head and neck paragangliomas: comparison of 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine, 18F-fluoro-2-deoxy-D-glucose PET, 123I-metaiodobenzylguanidine scintigraphy, and 111In-pentetreotide scintigraphy. J Clin Endocrinol Metab. 2011;96:2779–85. doi:10.1210/jc.2011-0333.
Gabriel S, Blanchet EM, Sebag F, Chen CC, Fakhry N, Deveze A, et al. Functional characterization of nonmetastatic paraganglioma and pheochromocytoma by (18)F-FDOPA PET: focus on missed lesions. Clin Endocrinol. 2013;79:170–7. doi:10.1111/cen.12126.
Chen CC, Czerwiec FS, Feuillan PP. Visualization of fibrous dysplasia during somatostatin receptor scintigraphy. J Nucl Med. 1998;39:238–40.
Agrawal K, Bhadada S, Mittal BR, Shukla J, Sood A, Bhattacharya A, et al. Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med. 2015;40:e6–10. doi:10.1097/RLU.0000000000000460.
Breer S, Brunkhorst T, Beil FT, Peldschus K, Heiland M, Klutmann S, et al. 68Ga DOTA-TATE PET/CT allows tumor localization in patients with tumor-induced osteomalacia but negative 111In-octreotide SPECT/CT. Bone. 2014;64:222–7. doi:10.1016/j.bone.2014.04.016.
Fottner C, Helisch A, Anlauf M, Rossmann H, Musholt TJ, Kreft A, et al. 6-18F-fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to 123I-metaiodobenzyl-guanidine scintigraphy in the detection of extraadrenal and hereditary pheochromocytomas and paragangliomas: correlation with vesicular monoamine transporter expression. J Clin Endocrinol Metab. 2010;95:2800–10.
Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, et al. 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab. 2009;94:3922–30.
Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–52. doi:10.1016/j.ccr.2013.04.018.
Saveanu A, Muresan M, De Micco C, Taieb D, Germanetti AL, Sebag F, et al. Expression of somatostatin receptors, dopamine D(2) receptors, noradrenaline transporters, and vesicular monoamine transporters in 52 pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2011;18:287–300. doi:10.1530/ERC-10-0175.
Kroiss A, Putzer D, Decristoforo C, Uprimny C, Warwitz B, Nilica B, et al. 68Ga-DOTA-TOC uptake in neuroendocrine tumour and healthy tissue: differentiation of physiological uptake and pathological processes in PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:514–23. doi:10.1007/s00259-012-2309-3.
Huang YT, Aziz SI, Ravi Kumar AS. Gallium-68 DOTA-TATE positron emission tomography/computed tomography: scintigraphic changes of adrenal glands following management of ectopic Cushing's syndrome by steroidogenesis inhibitors. World J Nucl Med. 2014;13:201–4. doi:10.4103/1450-1147.144823.
Varoquaux A, le Fur Y, Imperiale A, Reyre A, Montava M, Fakhry N, et al. Magnetic resonance spectroscopy of paragangliomas: new insights into in vivo metabolomics. Endocr Relat Cancer. 2015;22:M1–8. doi:10.1530/ERC-15-0246.
Blanchet EM, Millo C, Martucci V, Maass-Moreno R, Bluemke DA, Pacak K. Integrated whole-body PET/MRI with 18F-FDG, 18F-FDOPA, and 18F-FDA in paragangliomas in comparison with PET/CT: NIH first clinical experience with a single-injection, dual-modality imaging protocol. Clin Nucl Med. 2014;39:243–50. doi:10.1097/RLU.0000000000000289.
Hofman MS, Hicks RJ. Moving beyond "lumpology": PET/CT imaging of pheochromocytoma and paraganglioma. Clin Cancer Res. 2015;21:3815–7. doi:10.1158/1078-0432.CCR-15-1073.
Acknowledgments
This research was supported by the Intramural Research Program of the Assistance Publique-Hôpitaux de Marseille (AP-HM, France).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
Patient and tumor characteristics (location and uptake patterns). Negative on whole body acquisition, but positive on the craniocervical acquisition image (see Fig. 4). Quantitative results were obtained from WB images. VA visual analysis, JP jugular PGL, VP vagal PGL, LP laryngeal PGL, CBP carotid body PGL, PHEO pheochromocytoma, as apparently sporadic. (DOC 116 kb)
Rights and permissions
About this article
Cite this article
Archier, A., Varoquaux, A., Garrigue, P. et al. Prospective comparison of 68Ga-DOTATATE and 18F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. Eur J Nucl Med Mol Imaging 43, 1248–1257 (2016). https://doi.org/10.1007/s00259-015-3268-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3268-2