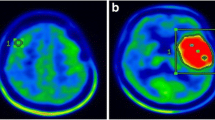
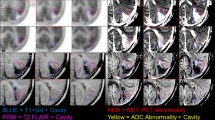
Abstract
Purpose
11C-methionine (MET) PET is an established diagnostic tool for glioma. Studies have suggested that MET uptake intensity in the tumor is a useful index for predicting patient outcome. Because MET uptake is known to reflect tumor expansion more accurately than MRI, we aimed to elucidate the association between volume-based tumor measurements and patient prognosis.
Methods
The study population comprised 52 patients with newly diagnosed glioma who underwent PET scanning 20 min after injection of 370 MBq MET. The tumor was contoured using a threshold of 1.3 times the activity of the contralateral normal cortex. Metabolic tumor volume (MTV) was defined as the total volume within the boundary. Total lesion methionine uptake (TLMU) was defined as MTV times the mean standardized uptake value (SUVmean) within the boundary. The tumor-to-normal ratio (TNR), calculated as the maximum standardized uptake value (SUVmax) divided by the contralateral reference value, was also recorded. All patients underwent surgery (biopsy or tumor resection) targeting the tissue with high MET uptake. The Kaplan-Meier method was used to estimate the predictive value of each measurement.
Results
Grade II tumor was diagnosed in 12 patients (3 diffuse astrocytoma, 2 oligodendroglioma, and 7 oligoastrocytoma), grade III in 18 patients (8 anaplastic astrocytoma, 6 anaplastic oligodendroglioma, and 4 anaplastic oligoastrocytoma), and grade IV in 22 patients (all glioblastoma). TNR, MTV and TLMU were 3.1 ± 1.2, 51.6 ± 49.9 ml and 147.7 ± 153.3 ml, respectively. None of the three measurements was able to categorize the glioma patients in terms of survival when all patients were analyzed. However, when only patients with astrocytic tumor (N = 33) were analyzed (i.e., when those with oligodendroglial components were excluded), MTV and TLMU successfully predicted patient outcome with higher values associated with a poorer prognosis (P < 0.05 and P < 0.01, respectively), while the predictive ability of TNR did not reach statistical significance (P = NS).
Conclusion
MTV and TLMU may be useful for predicting outcome in patients with astrocytic tumor.







Similar content being viewed by others
References
Rigau V, Zouaoui S, Mathieu-Daude H, Darlix A, Maran A, Tretarre B, et al. French brain tumor database: 5-year histological results on 25 756 cases. Brain Pathol. 2011;21:633–44. doi:10.1111/j.1750-3639.2011.00491.x.
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–31. doi:10.1016/s0140-6736(03)12328-8.
Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013;40:615–35. doi:10.1007/s00259-012-2295-5.
Becherer A, Karanikas G, Szabo M, Zettinig G, Asenbaum S, Marosi C, et al. Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30:1561–7. doi:10.1007/s00259-003-1259-1.
Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:176–82.
Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishiwata K, et al. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg. 2005;103:498–507. doi:10.3171/jns.2005.103.3.0498.
Pirotte B, Goldman S, Dewitte O, Massager N, Wikler D, Lefranc F, et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg. 2006;104:238–53. doi:10.3171/jns.2006.104.2.238.
Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49:694–9. doi:10.2967/jnumed.107.048082.
Okamoto S, Shiga T, Hattori N, Kubo N, Takei T, Katoh N, et al. Semiquantitative analysis of C-11 methionine PET may distinguish brain tumor recurrence from radiation necrosis even in small lesions. Ann Nucl Med. 2011;25:213–20. doi:10.1007/s12149-010-0450-2.
Singhal T, Narayanan TK, Jacobs MP, Bal C, Mantil JC. 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Nucl Med. 2012;53:1709–15. doi:10.2967/jnumed.111.102533.
Kaschten B, Stevenaert A, Sadzot B, Deprez M, Degueldre C, Del Fiore G, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med. 1998;39:778–85.
Kim S, Chung JK, Im SH, Jeong JM, Lee DS, Kim DG, et al. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2005;32:52–9. doi:10.1007/s00259-004-1598-6.
Ribom D, Eriksson A, Hartman M, Engler H, Nilsson A, Langstrom B, et al. Positron emission tomography (11)C-methionine and survival in patients with low-grade gliomas. Cancer. 2001;92:1541–9.
Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–27.
Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38. doi:10.1007/s00259-011-1934-6.
Chen HH, Chiu NT, Su WC, Guo HR, Lee BF. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264:559–66. doi:10.1148/radiol.12111148.
Ryu IS, Kim JS, Roh JL, Lee JH, Cho KJ, Choi SH, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis measured by 18F-FDG PET/CT in salivary gland carcinomas. J Nucl Med. 2013;54:1032–8. doi:10.2967/jnumed.112.116053.
Chu KP, Murphy JD, La TH, Krakow TE, Iagaru A, Graves EE, et al. Prognostic value of metabolic tumor volume and velocity in predicting head-and-neck cancer outcomes. Int J Radiat Oncol Biol Phys. 2012;83:1521–7. doi:10.1016/j.ijrobp.2011.10.022.
Kidd EA, Thomas M, Siegel BA, Dehdashti F, Grigsby PW. Changes in cervical cancer FDG uptake during chemoradiation and association with response. Int J Radiat Oncol Biol Phys. 2013;85:116–22. doi:10.1016/j.ijrobp.2012.02.056.
Liao S, Lan X, Cao G, Yuan H, Zhang Y. Prognostic predictive value of total lesion glycolysis from 18F-FDG PET/CT in post-surgical patients with epithelial ovarian cancer. Clin Nucl Med. 2013;38:715–20. doi:10.1097/RLU.0b013e31829f57fa.
Arbizu J, Tejada S, Marti-Climent JM, Diez-Valle R, Prieto E, Quincoces G, et al. Quantitative volumetric analysis of gliomas with sequential MRI and 11C-methionine PET assessment: patterns of integration in therapy planning. Eur J Nucl Med Mol Imaging. 2012;39:771–81. doi:10.1007/s00259-011-2049-9.
Lee IH, Piert M, Gomez-Hassan D, Junck L, Rogers L, Hayman J, et al. Association of 11C-methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:479–85. doi:10.1016/j.ijrobp.2008.04.050.
Galldiks N, Dunkl V, Kracht LW, Vollmar S, Jacobs AH, Fink GR, et al. Volumetry of [11C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol Imaging. 2012;11:516–27.
Fonti R, Larobina M, Del Vecchio S, De Luca S, Fabbricini R, Catalano L, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53:1829–35. doi:10.2967/jnumed.112.106500.
Kato T, Shinoda J, Oka N, Miwa K, Nakayama N, Yano H, et al. Analysis of 11C-methionine uptake in low-grade gliomas and correlation with proliferative activity. AJNR Am J Neuroradiol. 2008;29:1867–71. doi:10.3174/ajnr.A1242.
Manabe O, Hattori N, Yamaguchi S, Hirata K, Kobayashi K, Terasaka S, et al. Oligodendroglial component complicates the prediction of tumor grading with metabolic imaging. Eur J Nucl Med Mol Imaging. 2015. doi:10.1007/s00259-015-2996-7.
Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW. Volumetry of [11C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2010;37:84–92. doi:10.1007/s00259-009-1219-5.
Kawai N, Maeda Y, Kudomi N, Miyake K, Okada M, Yamamoto Y, et al. Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2011;38:441–50. doi:10.1007/s00259-010-1645-4.
Hirata K, Kobayashi K, Wong KP, Manabe O, Surmak A, Tamaki N, et al. A semi-automated technique determining the liver standardized uptake value reference for tumor delineation in FDG PET-CT. PLoS One. 2014;9:e105682. doi:10.1371/journal.pone.0105682.
Yamaguchi S, Kobayashi H, Hirata K, Shiga T, Tanaka S, Murata J, et al. Detection of histological anaplasia in gliomas with oligodendroglial components using positron emission tomography with (18)F-FDG and (11)C-methionine: report of two cases. J Neurooncol. 2011;101:335–41. doi:10.1007/s11060-010-0262-1.
Yoshikawa K, Kajiwara K, Morioka J, Fujii M, Tanaka N, Fujisawa H, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006;78:91–7. doi:10.1007/s11060-005-9064-2.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi:10.1007/s00401-007-0243-4.
Miyazaki M, Nishihara H, Terasaka S, Kobayashi H, Yamaguchi S, Ito T, et al. Immunohistochemical evaluation of O6-methylguanine DNA methyltransferase (MGMT) expression in 117 cases of glioblastoma. Neuropathology. 2014;34:268–76. doi:10.1111/neup.12091.
Smits A, Westerberg E, Ribom D. Adding 11C-methionine PET to the EORTC prognostic factors in grade 2 gliomas. Eur J Nucl Med Mol Imaging. 2008;35:65–71. doi:10.1007/s00259-007-0531-1.
Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol. 2008;10:1–18. doi:10.1007/s11307-007-0115-2.
Bading JR, Kan-Mitchell J, Conti PS. System A amino acid transport in cultured human tumor cells: implications for tumor imaging with PET. Nucl Med Biol. 1996;23:779–86.
Isselbacher KJ. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972;69:585–9.
Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–66. doi:10.1016/j.semcancer.2005.04.005.
Miwa K, Shinoda J, Yano H, Okumura A, Iwama T, Nakashima T, et al. Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: insight from a PET and MR fusion image study. J Neurol Neurosurg Psychiatry. 2004;75:1457–62. doi:10.1136/jnnp.2003.028480.
Ichimura K. Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol. 2012;29:131–9. doi:10.1007/s10014-012-0090-4.
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–9.
Brandes AA, Tosoni A, Cavallo G, Reni M, Franceschi E, Bonaldi L, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: a prospective GICNO study. J Clin Oncol. 2006;24:4746–53. doi:10.1200/jco.2006.06.3891.
Erdem-Eraslan L, Gravendeel LA, de Rooi J, Eilers PH, Idbaih A, Spliet WG, et al. Intrinsic molecular subtypes of glioma are prognostic and predict benefit from adjuvant procarbazine, lomustine, and vincristine chemotherapy in combination with other prognostic factors in anaplastic oligodendroglial brain tumors: a report from EORTC study 26951. J Clin Oncol. 2013;31:328–36. doi:10.1200/jco.2012.44.1444.
Salvati M, Formichella AI, D’Elia A, Brogna C, Frati A, Giangaspero F, et al. Cerebral glioblastoma with oligodendrogliomal component: analysis of 36 cases. J Neurooncol. 2009;94:129–34. doi:10.1007/s11060-009-9815-6.
Tanaka Y, Nariai T, Momose T, Aoyagi M, Maehara T, Tomori T, et al. Glioma surgery using a multimodal navigation system with integrated metabolic images. J Neurosurg. 2009;110:163–72. doi:10.3171/2008.4.17569.
Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, et al. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:64–74. doi:10.1016/j.ijrobp.2005.01.045.
Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii93–iii101. doi:10.1093/annonc/mdu050.
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–76; discussion 564–76. doi:10.1227/01.neu.0000317304.31579.17.
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. doi:10.3171/jns.2001.95.2.0190.
Suchorska B, Jansen NL, Linn J, Kretzschmar H, Janssen H, Eigenbrod S, et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84:710–9. doi:10.1212/wnl.0000000000001262.
Jansen NL, Suchorska B, Wenter V, Schmid-Tannwald C, Todica A, Eigenbrod S, et al. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med. 2015;56:9–15. doi:10.2967/jnumed.114.144675.
Acknowledgments
We thank Eriko Suzuki, Keiichi Magota, and Reiko Usui for their technical assistance.
Conflicts of interest
Funds were provided by the Translational Research Network Program of the Ministry of Education, Culture, Sports, Science and Technology (2014). Author K.H. has received a SNMMI Wagner-Torizuka Fellowship (2013/2015), Hokkaido University in conjunction with the HIROKO International Academic Exchange Foundation (2012), and Bayer Best Research Award of the Japan Radiological Society (2014).
Compliance with ethical standards
ᅟ
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Source of funding
Translational Research Network Program of Ministry of Education, Culture, Sports, Science and Technology (2014); SNMMI Wagner-Torizuka Fellowship (2013/2015); Hokkaido University HIROKO’s Fund for Academic Exchange (2012) Bayer Best Research Award of the Japan Radiological Society (2014).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(DOCX 164 kb)
Supplementary Figure 2
(DOCX 152 kb)
Supplementary Figure 3
(DOCX 150 kb)
Supplementary Table 1
(DOCX 15 kb)
Supplementary Table 2
(DOCX 15 kb)
Supplementary Table 3
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Kobayashi, K., Hirata, K., Yamaguchi, S. et al. Prognostic value of volume-based measurements on 11C-methionine PET in glioma patients. Eur J Nucl Med Mol Imaging 42, 1071–1080 (2015). https://doi.org/10.1007/s00259-015-3046-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3046-1