Abstract
Purpose
Activated microglia play a key role in inflammatory demyelinating injury in multiple sclerosis (MS). Microglial activation can be measured in vivo using a positron emission tomography (PET) ligand 11C-PBR28. We evaluated the test-retest variability (TRV) and lesion detectability of 11C-PBR28 binding in MS subjects and healthy controls (HCs) with high-resolution PET.
Methods
Four clinically and radiologically stable relapsing-remitting MS subjects (age 41 ± 7 years, two men/two women) and four HCs (age 42 ± 8 years, 2 two men/two women), matched for translocator protein genotype [two high- and two medium-affinity binders according to DNA polymorphism (rs6971) in each group], were studied for TRV. Another MS subject (age 41 years, male) with clinical and radiological activity was studied for lesion detectability. Dynamic data were acquired over 120 min after injection of 634 ± 101 MBq 11C-PBR28. For the TRV study, subjects were scanned twice, on average 1.4 weeks apart. Volume of distribution (V T) derived from multilinear analysis (MA1) modeling (t* = 30 min, using arterial input data) was the main outcome measure.
Results
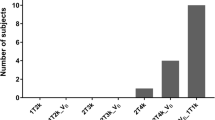
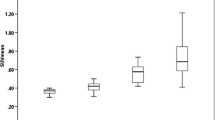
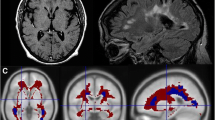
Mean test V T values (ml cm−3) were 3.9 ± 1.4 in the whole brain gray matter (GM), 3.6 ± 1.2 in the whole brain white matter (WM) or normal-appearing white matter (NAWM), and 3.3 ± 0.6 in MS WM lesions; mean retest V T values were 3.7 ± 1.0 in GM, 3.3 ± 0.9 in WM/NAWM, and 3.3 ± 0.7 in MS lesions. Test-retest results showed a mean absolute TRV ranging from 7 to 9 % across GM, WM/NAWM, and MS lesions. High-affinity binders demonstrated 30 % higher V T than medium-affinity binders in GM. Focal 11C-PBR28 uptake was detected in two enhancing lesions of the active MS patient.
Conclusion
High-resolution 11C-PBR28 PET can visualize focal areas where microglial activation is known to be present and has good test-retest reproducibility in the human brain. 11C-PBR28 PET is likely to be valuable for monitoring both MS disease evolution and response to therapeutic strategies that target microglial activation.







Similar content being viewed by others
References
Admas R, Vitor M, Ropper A. Principles of neurology. New York: McGraw-Hill; 1997.
Lladó X, Oliver A, Cabezas M, Freixenet J, Vilanova JC, Quiles A, et al. Segmentation of multiple sclerosis lesions in brain MRI: a review of automated approaches. Inf Sci 2012;186:164–85.
Compston A, Coles A. Multiple sclerosis. Lancet 2002;359:1221–31. doi:10.1016/S0140-6736(02)08220-X.
Kilpatrick TJ, Jokubaitis VG. Microglial function in MS pathology. In: Duncan ID, Franklin RJM, editors. Myelin repair and neuroprotection in multiple sclerosis. New York: Springer Science + Business Media; 2013.
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 2009;35:306–28. doi:10.1111/j.1365-2990.2008.01006.x.
Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, et al. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res 2007;1157:100–11. doi:10.1016/j.brainres.2007.04.054.
Choo IL, Carter SF, Schöll ML, Nordberg A. Astrocytosis measured by (11)C-deprenyl PET correlates with decrease in gray matter density in the parahippocampus of prodromal Alzheimer’s patients. Eur J Nucl Med Mol Imaging 2014;41:2120–6. doi:10.1007/s00259-014-2859-7.
Pagani M, Chio A, Valentini MC, Öberg J, Nobili F, Calvo A, et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 2014;83:1067–74. doi:10.1212/WNL.0000000000000792.
Setiawan E, Wilson A, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015;72:268–75. doi:10.1001/jamapsychiatry.2014.2427.
Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, et al. Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage 2010;49:2924–32. doi:10.1016/j.neuroimage.2009.11.056.
Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ. Influence of TSPO genotype on 11C-PBR28 standardized uptake values. J Nucl Med 2013;54:1320–2. doi:10.2967/jnumed.112.118885.
Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 2013;33:53–8. doi:10.1038/jcbfm.2012.131.
Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med 2011;52:24–32. doi:10.2967/jnumed.110.079459.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. doi:10.1002/ana.22366.
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 2012;32:1–5. doi:10.1038/jcbfm.2011.147.
Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(11)C]PBR28 PET study. Brain Behav Immun 2013;33:131–8. doi:10.1016/j.bbi.2013.06.010.
Carson RE, Barker WC, Liow J-S, Adler S, Johnson CA. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction of the HRRT. IEEE Nucl Sci Symp Med Imaging Conf. Portland, OR; 2003 M16-6.
Smith SM, De Stefano N, Jenkinson M, Matthews PM. Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr 2001;25:466–75.
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–89.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208–19. doi:10.1016/j.neuroimage.2004.07.051.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89. doi:10.1006/nimg.2001.0978.
Mowry EM, Beheshtian A, Waubant E, Goodin DS, Cree BA, Qualley P, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology 2009;72:1760–5. doi:10.1212/WNL.0b013e3181a609f8.
Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab 2002;22:1271–81. doi:10.1097/00004647-200210000-00015.
Giovacchini G, Lerner A, Toczek MT, Fraser C, Ma K, DeMar JC, et al. Brain incorporation of 11C-arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial-volume correction. J Nucl Med 2004;45:1471–9.
Müller-Gärtner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12:571–83. doi:10.1038/jcbfm.1992.81.
Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 2008;40:43–52. doi:10.1016/j.neuroimage.2007.11.011.
Collste KM. Test-retest reproducibility of C-11 PBR28 binding to TSPO in brain in control subjects. The 10th International Symposium on Functional NeuroReceptor Mapping of the Living Brain. The Netherlands; 2014. p. 102.
Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R, et al. Determination of [(11)C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab 2014;34:989–94. doi:10.1038/jcbfm.2014.84.
Jucaite A, Cselenyi Z, Arvidsson A, Ahlberg G, Julin P, Varnas K, et al. Kinetic analysis and test-retest variability of the radioligand [11C](R)-PK11195 binding to TSPO in the human brain - a PET study in control subjects. EJNMMI Res 2012;2:15. doi:10.1186/2191-219X-2-15.
Guo Q, Owen DR, Kalk NJ, Colasanti A, Weekes AA, Matthews PM, et al. Quantifying the specific TSPO signal of C-11 PBR28 in healthy humans: from in vitro to in vivo. International Symposium on Cerebral Blood flow, Metabolism and Function & International Conference on Quantification of Brain Function with PET. Shanghai, China; 2013. p. 126.
Guo Q, Owen DR, Rabiner EA, Turkheimer FE, Gunn RN. Identifying improved TSPO PET imaging probes through biomathematics: the impact of multiple TSPO binding sites in vivo. Neuroimage 2012;60:902–10. doi:10.1016/j.neuroimage.2011.12.078.
Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab 2010;30:1608–18. doi:10.1038/jcbfm.2010.63.
Guo Q, Colasanti A, Owen DR, Onega M, Kamalakaran A, Bennacef I, et al. Quantification of the specific translocator protein signal of 18F-PBR111 in healthy humans: a genetic polymorphism effect on in vivo binding. J Nucl Med 2013;54:1915–23. doi:10.2967/jnumed.113.121020.
Oh U, Fujita M, Ikonomidou VN, Evangelou IE, Matsuura E, Harberts E, et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol 2011;6:354–61. doi:10.1007/s11481-010-9243-6.
Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology 2007;68:634–42. doi:10.1212/01.wnl.0000250267.85698.7a.
Calabrese M, Atzori M, Bernardi V, Morra A, Romualdi C, Rinaldi L, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol 2007;254:1212–20. doi:10.1007/s00415-006-0503-6.
Chard D, Miller D. Grey matter pathology in clinically early multiple sclerosis: evidence from magnetic resonance imaging. J Neurol Sci 2009;282:5–11. doi:10.1016/j.jns.2009.01.012.
Chataway J, Schuerer N, Alsanousi A, Chan D, Macmanus D, Hunter K, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet 2014;383(9936):2213–21. doi:10.1016/S0140-6736(13)62242-4.
De Stefano N, Guidi L, Stromillo ML, Bartolozzi ML, Federico A. Imaging neuronal and axonal degeneration in multiple sclerosis. Neurol Sci 2003;24 Suppl 5:S283–6. doi:10.1007/s10072-003-0175-2.
Sanfilipo MP, Benedict RH, Sharma J, Weinstock-Guttman B, Bakshi R. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage 2005;26:1068–77. doi:10.1016/j.neuroimage.2005.03.008.
Tedeschi G, Lavorgna L, Russo P, Prinster A, Dinacci D, Savettieri G, et al. Brain atrophy and lesion load in a large population of patients with multiple sclerosis. Neurology 2005;65:280–5. doi:10.1212/01.wnl.0000168837.87351.1f.
Dickstein LP, Zoghbi SS, Fujimura Y, Imaizumi M, Zhang Y, Pike VW, et al. Comparison of 18F- and 11C-labeled aryloxyanilide analogs to measure translocator protein in human brain using positron emission tomography. Eur J Nucl Med Mol Imaging 2011;38(2):352–7. doi:10.1007/s00259-010-1622-y.
Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 2013;136(Pt 7):2228–38. doi:10.1093/brain/awt145.
Abi-Dargham A, Gandelman M, Zoghbi SS, Laruelle M, Baldwin RM, Randall P, et al. Reproducibility of SPECT measurement of benzodiazepine receptors in human brain with iodine-123-iomazenil. J Nucl Med 1995;36(2):167–75.
Acknowledgments
We thank the staff at Yale PET Center, Yale-New Haven Hospital, and Yale MR Research Center. This study was supported by F. Hoffmann-La Roche Ltd and SNMMI research grant for junior medical faculty.
Compliance with ethical standards
ᅟ
Conflicts of interest
Kevin C. O’Connor has received speaker honoraria from EMD-Serono. Nicholas Seneca and David Leppert are currently employees of Hoffmann-La Roche Ltd. Daniel Pelletier received consulting honoraria from CNS Imaging Consultant, LLC. Eunkyung Park, Jean-Dominique Gallezot, Aracely Delgadillo, Shuang Liu, Beata Planeta, Shu-Fei Lin, Keunpoong Lim, Jae-Yun Lee, Anne Chastre, Ming-Kai Chen, Yiyun Huang, and Richard E. Carson have no conflict of interest relevant to this article.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, E., Gallezot, JD., Delgadillo, A. et al. 11C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur J Nucl Med Mol Imaging 42, 1081–1092 (2015). https://doi.org/10.1007/s00259-015-3043-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3043-4