Abstract
Purpose
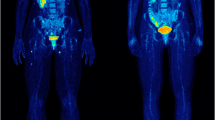
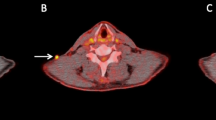
Ipilimumab is a newly approved immunotherapeutic agent that has been shown to provide a survival benefit in patients with metastatic melanoma. 18F-FDG PET/CT has demonstrated very satisfying results in detecting melanoma metastases in general. Using 18F-FDG PET/CT we monitored patients with metastatic melanoma undergoing ipilimumab therapy during the course of treatment. The aim of our study was to evaluate the role of 18F-FDG PET/CT performed after two cycles of ipilimumab in predicting the final response to therapy.
Methods
In 22 patients suffering from unresectable metastatic melanoma, scheduled for ipilimumab treatment PET/CT scanning was performed before the start of treatment (baseline scan), after two cycles of treatment (early response) and at the end of treatment after four cycles (late response). Evaluation of the patient response to treatment on PET was based on the European Organization for Research and Treatment of Cancer 1999 criteria. Progression-free survival (PFS) and overall survival (OS) data are presented.
Results
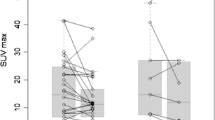
After the end of treatment, 15 patients were characterized as having progressive metabolic disease (PMD) and five as having stable metabolic disease (SMD), and two patients showed a partial metabolic response (PMR). Early PET/CT performed after two ipilimumab cycles predicted treatment response in 13 of the 15 PMD patients, in five of the five SMD patients and in neither of the two PMR patients. Both patients with PMR showed pseudoprogression after the second cycle and were therefore wrongly classified. According to the patients’ clinical outcome, patients with late PMD had a median PFS of 3.6 months (mean 5.6 months), while patients with late SMD had a median PFS of 9.8 months (mean 9.0 months). In comparison, patients with early PMD had a median PFS of 2.7 months (mean 5.5 months) and patients with early SMD had a median PFS of 6.3 months (mean 7.5 months). The difference in PFS between the two groups was statistically significant for both early and late responses (log-rank p < 0.001). Median OS among patients with late PMD was 9.1 months (mean 11.2 months) and among those with late SMD 9.8 months (mean 10.7 months). The difference in OS between the two groups was statistically significant (log-rank p = 0.013). The median OS among patients with early PMD was 8.8 months (mean 12.0 months) and among those with early SMD 9.8 months (mean 10.0 months). The difference in OS between the two groups was statistically significant (log-rank p < 0.001).
Conclusion
18F-FDG PET/CT after two cycles of ipilimumab is highly predictive of the final treatment outcome in patients with PMD and SMD.






Similar content being viewed by others
References
Fellner C. Ipilimumab (Yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P T. 2012;37:503–30.
World Health Organization. Skin cancers. Available at: http://www.who.int/uv/faq/skincancer/en/index1.html. Accessed 25 Oct 2014.
National Cancer Institute. SEER stat fact sheets: melanoma of the skin. Available at: http://seer.cancer.gov/statfacts/html/melan.html. Accessed 25 Oct 2014.
International Agency for Research on Cancer. World Health Organization. Malignant melanoma of skin. Available at: http://eu-cancer.iarc.fr/EUCAN/Cancer.aspx?Cancer=20. Accessed 25 Oct 2014.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;368:711–23.
Lawrence DP, Rubin KM. Melanoma. In: Chabner BA, Lynch Jr TJ, Longo DL, editors. Harrison's manual of oncology. New York: McGraw Hill; 2008. p. 537–48.
Eggermont AM, Kirkwood JM. Reevaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–36.
Petrella T, Quirt I, Verma S, Haynes AE, Charette M, Bak K, et al. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev. 2007;33:484–96.
Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26.
Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G; ESMO Guidelines Working Group. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii86–91.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Melanoma Version 2.2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed 23 Jan 2014.
Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily – CTLA-4. Nature. 1987;328:267–70.
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20.
Dietlein M, Krug B, Groth W, Smolarz K, Scheidhauer K, Psaras T, et al. Positron emission tomography using 18F-fluorodeoxyglucose in advanced stages of malignant melanoma: a comparison of ultrasonographic and radiological methods of diagnosis. Nucl Med Commun. 1999;20:255–61.
Fuster D, Chiang S, Johnson G, Schuchter LM, Zhuang H, Alavi A. Is 18F-FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma? J Nucl Med. 2004;45:1323–27.
Cancer Council Australia/Australian Cancer Network/Ministry of Health, New Zealand. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand; 2008. Available at http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp111.pdf. Accessed 25 Oct 2014.
Holder Jr WD, White Jr RL, Zuger JH, Easton Jr EJ, Greene FL. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–69. discussion 769–771.
Swetter SM, Carroll LA, Johnson DL, Segall GM. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646–53.
Schauwecker DS, Siddiqui AR, Wagner JD, Davidson D, Jung SH, Carlson KA, et al. Melanoma patients evaluated by four different positron emission tomography reconstruction techniques. Nucl Med Commun. 2003;24:281–89.
Mijnhout GS, Hoekstra OS, van Tulder MW, Teule GJ, Devillé WL. Systematic review of the diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer. 2001;91:1530–42.
Medicare National Coverage Determinations Manual, Section 220.6. Available at https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/ncd103c1_Part4.pdf. Accessed 25 Oct 2014.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–82.
Blodgett TM. Brain metastases. In: Blodgett TM, Ryan A, Almusa O, Papachristou M, Paidisetty S, editors. Specialty imaging. PET/CT oncologic imaging with correlative diagnostic CT. Salt Lake City: Amirsys; 2009. p. 14–22.
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–48.
Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C. A Java environment for medical image data analysis: initial application for brain PET quantitation. Med Inform (Lond). 1998;23:207–14.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein C, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
Ribas A, Benz MR, Allen-Auerbach MS, Radu C, Chmielowski B, Seja E, et al. Imaging of CTLA4 blockade-induced cell replication with 18F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med. 2010;51:340–46.
de Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–98.
Schaefer NG, Veit-Haibach P, Soyka JD, Steinert HC, Stahel RA. Continued pemetrexed and platin-based chemotherapy in patients with malignant pleural mesothelioma (MPM): value of 18F-FDG-PET/CT. Eur J Radiol. 2012;81:e19–25.
Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer. 2003;39:2012–20.
Zerizer I, Al-Nahhas A, Towey D, Tait P, Ariff B, Wasan H, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging. 2012;39:1391–99.
Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15:7116–18.
Weber JS, O'Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–56.
Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117.
Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–306.
Wilgenhof S, Du Four S, Everaert H, Neyns B. Patterns of response in patients with pretreated metastatic melanoma who received ipilimumab 3 mg/kg in a European expanded access program: five illustrative case reports. Cancer Investig. 2012;30:712–20.
Kang HC, Kim CY, Han JH, Choe GY, Kim JH, Kim JH, et al. Pseudoprogression in patients with malignant gliomas treated with concurrent temozolomide and radiotherapy: potential role of p53. J Neurooncol. 2011;102:157–62.
Konstantinou MP, Dutriaux C, Gaudy-Marqueste C, Mortier L, Bedane C, Girard C, et al. Ipilimumab in melanoma patients with brain metastasis: a retrospective multicentre evaluation of 38 patients. Acta Derm Venereol. 2014;94:45–9.
da Cruz LC Jr H, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32:1978–85.
Skougaard K, Nielsen D, Jensen BV, Hendel HW. Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med. 2013;54:1026–31.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Antonia Dimitrakopoulou-Strauss and Jessica C. Hassel contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sachpekidis, C., Larribere, L., Pan, L. et al. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging 42, 386–396 (2015). https://doi.org/10.1007/s00259-014-2944-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2944-y