Abstract
Purpose
[18F]Fluoro-3′-deoxy-3′-l-fluorothymidine ([18F]FLT) is a tissue proliferation marker which has been widely validated as a tumour-specific imaging tracer for PET. [18F]FLT uptake in breast cancer is generally quantified at the region level or through first-order statistical descriptors (mean or maximum value), approaches that ignore the known complexity and heterogeneity of cancer tissues. Our aims were: (1) to validate a robust and reproducible voxel-wise approach to the quantification of [18F]FLT PET data in breast cancer patients, and (2) to exploit the entire distribution of the [18F]FLT retention estimates and their variability in the tumour region for the prediction of early treatment response.
Methods
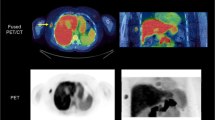
The dataset was derived from 15 patients with stage II–IV breast cancer, scanned twice before chemotherapy and once 1 week after therapy. Using RECIST criteria (after 60 days) nine patients were categorized as responders or nonresponders to treatment. Kinetic modelling (compartmental modelling, Patlak analysis and spectral analysis with iterative filter), tissue-to-plasma ratio and standardized uptake value were applied at the voxel level. Test–retest estimates were used to assess reproducibility and reliability of the [18F]FLT uptake values before and after therapy for responder/nonresponder prediction.
Results
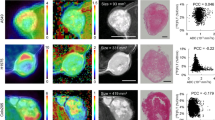
All the methods provided a measure of [18F]FLT uptake that was reliable and reproducible with ICC >0.94. Moreover, a very strong correlation was found among the methods (R 2 > 0.81). All the methods provided a limited number of outliers (<20 % in tumour), with the exception of compartmental modelling (>25 %) which was therefore excluded from the prediction analysis. Differences between before and after therapy in mean voxel-wise uptake in tumour did not allow a complete responder/nonresponder classification. In contrast, considering the full estimate distributions within the tumour (changes in median and mode between before and after therapy) improved therapy response for all the analysed methods.
Conclusion
We showed that kinetic modelling (Patlak and spectral analysis with iterative filter) applied voxel-wise allows appropriate [18F]FLT uptake estimation in breast cancer with good reproducibility. Notably, this study indicated that a more comprehensive statistical investigation could improve tumour characterization and prediction of treatment response.







Similar content being viewed by others
References
Been LB, Suurmeijer AJH, Cobben DCP, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging. 2004;31(12):1659–72. doi:10.1007/s00259-004-1687-6.
Shields AF, Grierson JR, Kozawa SM, Zheng M. Development of labeled thymidine analogs for imaging tumor proliferation. Nucl Med Biol. 1996;23(1):17–22.
Ullrich R, Backes H, Li H, Kracht L, Miletic H, Kesper K, et al. Glioma proliferation as assessed by 3'-fluoro-3'-deoxy-L-thymidine positron emission tomography in patients with newly diagnosed high-grade glioma. Clin Cancer Res. 2008;14(7):2049–55.
Kenny LM. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 2005;65(21):10104–12. doi:10.1158/0008-5472.can-04-4297.
Buck AK, Hetzel M, Schirrmeister H, Halter G, Möller P, Kratochwil C, et al. Clinical relevance of imaging proliferative activity in lung nodules. Eur J Nucl Med Mol Imaging. 2005;32(5):525–33.
Buck AK, Bommer M, Stilgenbauer S, Juweid M, Glatting G, Schirrmeister H, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66(22):11055–61.
Salskov A, Tammisetti VS, Grierson J, Vesselle H. FLT: measuring tumor cell proliferation in vivo with positron emission tomography and 3'-deoxy-3'-[18F]fluorothymidine. Semin Nucl Med. 2007;37(6):429–39.
Visvikis D, Francis D, Mulligan R, Costa D, Croasdale I, Luthra S, et al. Comparison of methodologies for the in vivo assessment of 18FLT utilisation in colorectal cancer. Eur J Nucl Med Mol Imaging. 2004;31(2):169–78.
Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45(9):1431–4.
Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34(9):1339–47. doi:10.1007/s00259-007-0379-4.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3(1):1–7.
Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13(1):15–23.
Muzi M, Mankoff DA, Grierson JR, Wells JM, Vesselle H, Krohn KA. Kinetic modeling of 3′-deoxy-3′-fluorothymidine in somatic tumors: mathematical studies. J Nucl Med. 2005;46(2):371–80.
Muzi M, Vesselle H, Grierson JR, Mankoff DA, Schmidt RA, Peterson L, et al. Kinetic analysis of 3′-deoxy-3′-fluorothymidine PET studies: validation studies in patients with lung cancer. J Nucl Med. 2005;46(2):274–82.
Harris RJ, Cloughesy TF, Pope WB, Nghiemphu PL, Lai A, Zaw T, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14(8):1079–89.
Laymon CM, Oborski MJ, Lee VK, Davis DK, Wiener EC, Lieberman FS, et al. Combined imaging biomarkers for therapy evaluation in glioblastoma multiforme: correlating sodium MRI and F-18 FLT PET on a voxel-wise basis. Magn Reson Imaging. 2012;30(9):1268–78.
Nyflot MJ, Harari PM, Yip S, Perlman SB, Jeraj R. Correlation of PET images of metabolism, proliferation and hypoxia to characterize tumor phenotype in patients with cancer of the oropharynx. Radiother Oncol. 2012;105(1):36–40.
Willaime JM, Turkheimer FE, Kenny LM, Aboagye EO. Quantification of intra-tumour cell proliferation heterogeneity using imaging descriptors of 18F fluorothymidine-positron emission tomography. Phys Med Biol. 2013;58(2):187–203. doi:10.1088/0031-9155/58/2/187.
Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805(1):105–17.
Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38(6):987–91.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–16.
Cleij MC, Steel CJ, Brady F, Ell PJ, Pike VW, Luthra SK. An improved synthesis of 3′‐DEOXY‐3′‐[18F]fluorothymidine ([18F]FLT) and the fate of the precursor, 2,3′‐anhydro‐5′-O-(4,4′‐dimethoxytrityl)‐thymidine. J Label Compd Radiopharm. 2001;44(S1):S871–3.
Veronese M, Bertoldo A, Bishu S, Unterman A, Tomasi G, Smith CB, et al. A spectral analysis approach for determination of regional rates of cerebral protein synthesis with the L-[1-(11)C]leucine PET method. J Cereb Blood Flow Metab. 2010;30(8):1460–76.
Lehtiö K, Oikonen V, Nyman S, Grönroos T, Roivainen A, Eskola O, et al. Quantifying tumour hypoxia with fluorine-18 fluoroerythronitroimidazole ([18F]FETNIM) and PET using the tumour to plasma ratio. Eur J Nucl Med Mol Imaging. 2003;30(1):101–8. doi:10.1007/s00259-002-1016-x.
van den Hoff J, Oehme L, Schramm G, Maus J, Lougovski A, Petr J, et al. The PET-derived tumor-to-blood standard uptake ratio (SUR) is superior to tumor SUV as a surrogate parameter of the metabolic rate of FDG. EJNMMI Res. 2013;3(1):77.
Kong X, Zhu Q, Vidal P, Watanabe K, Polsky B, Armstrong D, et al. Comparisons of anti-human immunodeficiency virus activities, cellular transport, and plasma and intracellular pharmacokinetics of 3′-fluoro-3′-deoxythymidine and 3′-azido-3′-deoxythymidine. Antimicrob Agents Chemother. 1992;36(4):808–18.
Gazziola C, Ferraro P, Moras M, Reichard P, Bianchi V. Cytosolic high K(m) 5′-nucleotidase and 5′(3′)-deoxyribonucleotidase in substrate cycles involved in nucleotide metabolism. J Biol Chem. 2001;276(9):6185–90.
Grierson JR, Schwartz JL, Muzi M, Jordan R, Krohn KA. Metabolism of 3′-deoxy-3′-[F-18] fluorothymidine in proliferating A549 cells: validations for positron emission tomography. Nucl Med Biol. 2004;31(7):829–37.
Veronese M, Schmidt KC, Smith CB, Bertoldo A. Use of spectral analysis with iterative filter for voxelwise determination of regional rates of cerebral protein synthesis with L-[1-11C]leucine PET. J Cereb Blood Flow Metab. 2012;32(6):1073–85.
Veronese M, Rizzo G, Turkheimer FE, Bertoldo A. SAKE: a new quantification tool for positron emission tomography studies. Comput Methods Programs Biomed. 2013;111(1):199–213.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50 Suppl 1:11S–20S. doi:10.2967/jnumed.108.057182.
Grecchi E, Veronese M, Moresco RM, Bellani G, Pesenti A, Messa C, et al. Assessment of voxelwise quantification of [18F]FDG dynamic PET data in human lung: insight for clinical use. Proceedings of the 2012 World Molecular Imaging Congress, Dublin, Ireland, 5–8 September 2012.
Mazoyer BM, Huesman RH, Budinger TF, Knittel BL. Dynamic PET data analysis. J Comput Assist Tomogr. 1986;10(4):645–53.
Bertoldo A, Vicini P, Sambuceti G, Lammertsma AA, Parodi O, Cobelli C. Evaluation of compartmental and spectral analysis models of [18F]FDG kinetics for heart and brain studies with PET. IEEE Trans Biomed Eng. 1998;45(12):1429–48. doi:10.1109/10.730437.
Acknowledgement
The authors would like to thank Dr. Julien Williem for his help and support in organizing the dataset.
This research was supported in part by UK Medical Research Council (core grant MC_A652_5PY80, and programme grant “Quantitative methodologies for Positron Emission Tomography”, no. G1100809/1). Portions of this work were presented in preliminary form at International Society of Nuclear Medicine and Molecular Imaging meeting (Vancouver, 2013).
Conflicts on interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Veronese and G. Rizzo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Veronese, M., Rizzo, G., Aboagye, E.O. et al. Parametric imaging of 18F-fluoro-3-deoxy-3-l-fluorothymidine PET data to investigate tumour heterogeneity. Eur J Nucl Med Mol Imaging 41, 1781–1792 (2014). https://doi.org/10.1007/s00259-014-2757-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2757-z