Abstract
Purpose
Human epidermal growth factor receptor type 3 (HER3) is a transmembrane receptor tyrosine kinase belonging to the HER (ErbB) receptor family. Membranous expression of HER3 is associated with trastuzumab resistance in breast cancer and the transition to androgen independence in prostate cancer. Imaging of HER3 expression in malignant tumors may provide important diagnostic information that can influence patient management. Affibody molecules with low picomolar affinity to HER3 were recently selected. The aim of this study was to investigate the feasibility of HER3 imaging using radiolabeled Affibody molecules.
Methods
A HER3-binding Affibody molecule, Z08699, with a HEHEHE-tag on N-terminus was labeled with 99mTc(CO)3 using an IsoLink kit. In vitro and in vivo binding specificity and the cellular processing of the labeled binder were evaluated. Biodistribution of 99mTc(CO)3-HEHEHE-Z08699 was studied over time in mice bearing HER3-expressing xenografts.
Results
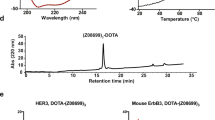
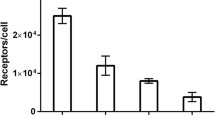
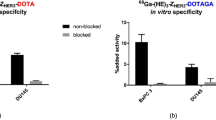
HEHEHE-Z08699 was labeled with 99mTc(CO)3 with an isolated yield of >80 % and a purity of >99 %. Binding of 99mTc(CO)3-HEHEHE-Z08699 was specific to BT474 and MCF7 (breast cancer), and LS174T (colon cancer) cells. Cellular processing showed rapid binding and relatively quick internalization of the receptor/Affibody molecule complex (70 % of cell-associated radioactivity was internalized after 24 h). The tumor targeting was receptor mediated and the excretion was predominantly renal. Receptor-mediated uptake was also found in the liver, lung, stomach, intestine, and salivary glands. At 4 h pi, tumor-to-blood ratios were 7 ± 3 for BT474, and 6 ± 2 for LS174T xenografts. LS174T tumors were visualized by microSPECT 4 h pi.
Conclusions
The results of this study suggest the feasibility of HER3-imaging in malignant tumors using Affibody molecules.






Similar content being viewed by others
References
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58:903–13.
Ocaña A, Pandiella A. Targeting HER receptors in cancer. Curr Pharm Des. 2013;19:808–17.
Aurisicchio L, Marra E, Roscilli G, Mancini R, Ciliberto G. The promise of anti-ErbB3 monoclonals as new cancer therapeutics. Oncotarget. 2012;3:744–58.
Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453–7.
Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75.
Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sánchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108:5021–6.
Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res. 2010;316:1083–100.
Servidei T, Riccardi A, Mozzetti S, Ferlini C, Riccardi R. Chemoresistant tumor cell lines display altered epidermal growth factor receptor and HER3 signaling and enhanced sensitivity to gefitinib. Int J Cancer. 2008;123:2939–49.
Jathal MK, Chen L, Mudryj M, Ghosh PM. Targeting ErbB3: the New RTK(id) on the Prostate Cancer Block. Immunol Endocr Metab Agents Med Chem. 2011;11:131–49.
Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst. 2013;105:266–73.
Tolmachev V, Stone-Elander S, Orlova A. Radiolabelled receptor-tyrosine-kinase targeting drugs for patient stratification and monitoring of therapy response: prospects and pitfalls. Lancet Oncol. 2010;11:992–1000.
van Dongen GA, Poot AJ, Vugts DJ. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: immuno-PET and TKI-PET. Tumour Biol. 2012;33:607–15.
van Dongen GA, Visser GW, Lub-de Hooge MN, de Vries EG, Perk LR. Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist. 2007;12:1379–89.
Wester HJ, Kessler H. Molecular targeting with peptides or peptide-polymer conjugates: just a question of size? J Nucl Med. 2005;46:1940–5.
Löfblom J, Feldwisch J, Tolmachev V, Carlsson J, Ståhl S, Frejd FY. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010;584:2670–80.
Orlova A, Magnusson M, Eriksson TL, Nilsson M, Larsson B, Höidén-Guthenberg I, et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006;66:4339–48.
Tolmachev V, Rosik D, Wållberg H, Sjöberg A, Sandström M, Hansson M, et al. Imaging of EGFR expression in murine xenografts using site-specifically labelled anti-EGFR 111In-DOTA-Z EGFR:2377 Affibody molecule: aspect of the injected tracer amount. Eur J Nucl Med Mol Imaging. 2010;37:613–22.
Tolmachev V, Malmberg J, Hofström C, Abrahmsén L, Bergman T, Sjöberg A, et al. Imaging of IGF-1R in prostate cancer xenografts using the Affibody molecule 111In-DOTA-Z IGF1R:4551. J Nucl Med. 2012;53:146–53.
Ahlgren S, Orlova A, Rosik D, Sandström M, Sjöberg A, Baastrup B, et al. Evaluation of Maleimide Derivative of DOTA for Site-Specific Labeling of Recombinant Affibody Molecules. Bioconjug Chem. 2008;19:235–43.
Friedman M, Orlova A, Johansson E, Eriksson T, Höidén-Guthenberg I, Tolmachev V, et al. Directed evolution to low nanomolar affinity of an epidermal growth factor receptor 1-binding Affibody molecule. J Mol Biol. 2008;376:1388–402.
Tolmachev V, Tran TA, Rosik D, Abrahmsén L, Sjöberg A, Orlova A. Tumor targeting using Affibody molecules: an interplay of a target expression level, affinity and binding site composition. J Nucl Med. 2012;53:953–60.
Baum RP, Prasad V, Müller D, Schuchardt C, Orlova A, Wennborg A, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med. 2010;51:892–7.
Sörensen J, Sandberg D, Carlsson J, Sandström M, Wennborg A, Feldwisch J, Tolmachev V, Åström G, Lubberink M, Garske-Roman U, Lindman H. First-in-Human Molecular Imaging of HER2 Expression in Breast Cancer Metastases Using the 111In-ABY-025 Affibody Molecule. J Nucl Med, 2014, in press.
Kronqvist N, Malm M, Göstring L, Gunneriusson E, Nilsson M, Höidén Guthenberg I, et al. Combining phage and staphylococcal surface display for generation of ErbB3-specific Affibody molecules. Protein Eng Des Sel. 2011;24:385–96.
Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99:1415–25.
Löfblom J. Bacterial display in combinatorial protein engineering. Biotechnol J. 2011;6:1115–29.
Malm M, Kronqvist N, Lindberg H, Gudmundsdotter L, Bass T, Frejd FY, et al. Inhibiting HER3-mediated tumor cell growth with Affibody molecules engineered to low picomolar affinity by position-directed error-prone PCR-like diversification. PLoS One. 2013;8:e62791.
Tolmachev V, Hofström C, Malmberg J, Ahlgren S, Hosseinimehr SJ, Sandström M, et al. HEHEHE-tagged affibody molecule may be purified by IMAC, is conveniently labeled with [99(m)Tc(CO)3](+), and shows improved biodistribution with reduced hepatic radioactivity accumulation. Bioconjug Chem. 2010;21:2013–22.
Hofström C, Altai M, Honarvar H, Strand J, Malmberg J, Hosseinimehr SJ, et al. HAHAHA, HEHEHE, HIHIHI or HKHKHK: influence of position and composition of histidine containing tags on biodistribution of [99mTc(CO)3] + - labeled affibody molecules. J Med Chem. 2013;56:4966–74.
Waibel R, Alberto R, Willuda J, et al. Stable one-step technetium-99 m labeling of His-tagged recombinant proteins with a novel Tc(I)-carbonyl complex. Nat Biotechnol. 1999;17:897–901.
Yoshioka T, Nishikawa Y, Ito R, Kawamata M, Doi Y, Yamamoto Y, et al. Significance of integrin αvβ5 and erbB3 in enhanced cell migration and liver metastasis of colon carcinomas stimulated by hepatocyte-derived heregulin. Cancer Sci. 2010;101:2011–8.
Janmaat ML, Rodriguez JA, Jimeno J, Kruyt FA, Giaccone G. Kahalalide F induces necrosis-like cell death that involves depletion of ErbB3 and inhibition of Akt signaling. Mol Pharmacol. 2005;68:502–10.
Kimura F, Iwaya K, Kawaguchi T, Kaise H, Yamada K, Mukai K, et al. Epidermal growth factor-dependent enhancement of invasiveness of squamous cell carcinoma of the breast. Cancer Sci. 2010;101:1133–40.
Wållberg H, Orlova A. Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: implications for development of labeled tracers. Cancer Biother Radiopharm. 2008;23:435–42.
Tolmachev V, Wållberg H, Sandström M, Hansson M, Wennborg A, Orlova A. Optimal specific radioactivity of anti-HER2 Affibody molecules enables discrimination between xenografts with high and low HER2 expression levels. Eur J Nucl Med Mol Imaging. 2011;38:531–9.
Orlova A, Nilsson F, Wikman M, Widström C, Ståhl S, Carlsson J, et al. Comparative in vivo evaluation of technetium and iodine labels on an anti-HER2 Affibody for single-photon imaging of HER2 expression in tumors. J Nucl Med. 2006;47:512–9.
Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandström M, et al. Synthetic Affibody Molecules: A Novel Class of Affinity Ligands for Molecular Imaging of HER2-Expressing Malignant Tumors. Cancer Res. 2007;67:2178–86.
Tolmachev V, Varasteh Z, Honarvar H, Eriksson O, Jonasson P, Frejd FY, Abrahmsen L, Orlova A. Imaging of PDGFRβ expression in gliobalstoma xenografts using Affibody molecule 111In-DOTA-Z09591. J Nucl Med, 2014;55:294–300.
Orlova A, Hofström C, Strand J, Varasteh Z, Sandstrom M, Andersson K, et al. [99mTc(CO)3]+-(HE)3-ZIGF1R:4551, a new affibody conjugate for visualization of insulin-like growth factor 1 receptor expression in malignant tumours. Eur J Nucl Med Mol Imaging. 2013;40(3):439–49.
Tolmachev V. Imaging of HER-2 overexpression in tumors for guiding therapy. Curr Pharm Des. 2008;14:2999–3019.
Acknowledgments
This work was supported by grants from the Swedish Cancer Society (Cancerfonden) and the Swedish Research Council (Vetenskapsrådet).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 109 kb)
Rights and permissions
About this article
Cite this article
Orlova, A., Malm, M., Rosestedt, M. et al. Imaging of HER3-expressing xenografts in mice using a 99mTc(CO)3-HEHEHE-ZHER3:08699 affibody molecule. Eur J Nucl Med Mol Imaging 41, 1450–1459 (2014). https://doi.org/10.1007/s00259-014-2733-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2733-7