Abstract
Purpose
Diagnosis of tauopathies such as Alzheimer’s disease (AD) still relies on post-mortem examination of the human brain. A non-invasive method of determining brain tau burden in vivo would allow a better understanding of the pathophysiology of tauopathies. The purpose of the study was to evaluate 18F-THK523 as a potential tau imaging tracer.
Methods
Ten healthy elderly controls, three semantic dementia (SD) and ten AD patients underwent neuropsychological examination, MRI as well as 18F-THK523 and 11C-Pittsburgh compound B (PIB) positron emission tomography (PET) scans. Composite memory and non-memory scores, global and hippocampal brain volume, and partial volume-corrected tissue ratios for 18F-THK523 and 11C-PIB were estimated for all participants. Correlational analyses were performed between global and regional 18F-THK523, 11C-PIB, cognition and brain volumetrics.
Results
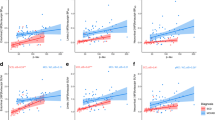
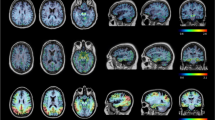
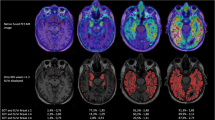
18F-THK523 presented with fast reversible kinetics. Significantly higher 18F-THK523 retention was observed in the temporal, parietal, orbitofrontal and hippocampi of AD patients when compared to healthy controls and SD patients. White matter retention was significantly higher than grey matter retention in all participants. The pattern of cortical 18F-THK523 retention did not correlate with Aβ distribution as assessed by 11C-PIB and followed the known distribution of tau in the AD brain, being higher in temporal and parietal areas than in the frontal region. Unlike 11C-PIB, hippocampal 18F-THK523 retention was correlated with several cognitive parameters and with hippocampal atrophy.
Conclusion
18F-THK523 does not bind to Aβ in vivo, while following the known distribution of paired helical filaments (PHF)-tau in the brain. Significantly higher cortical 18F-THK523 retention in AD patients as well as the association of hippocampal 18F-THK523 retention with cognitive parameters and hippocampal volume suggests 18F-THK523 selectively binds to tau in AD patients. Unfortunately, the very high 18F-THK523 retention in white matter precludes simple visual inspection of the images, preventing its use in research or clinical settings.



Similar content being viewed by others
References
Masters CL, Cappai R, Barnham KJ, Villemagne VL. Molecular mechanisms for Alzheimer’s disease: implications for neuroimaging and therapeutics. J Neurochem 2006;97(6):1700–25. doi:10.1111/j.1471-4159.2006.03989.x.
Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs 2010;24(5):375–98. doi:10.2165/11533100-000000000-00000.
Mohorko N, Bresjanac M. Tau protein and human tauopathies: an overview. Zdrav Vestn 2008;77(Suppl II):35–41.
Komori T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol 1999;9(4):663–79.
McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68(7):709–35. doi:10.1097/NEN.0b013e3181a9d503.
Delacourte A. Tauopathies: recent insights into old diseases. Folia Neuropathol 2005;43(4):244–57.
Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 2000;33(1):95–130.
Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer’s disease. Brain Pathol 2007;17(1):83–90. doi:10.1111/j.1750-3639.2007.00053.x.
Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl 1998;53:127–40.
Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995;16(3):271–8. discussion 278–84.
Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 1999;52(6):1158–65.
Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta 2005;1739(2–3):240–50.
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 2007;316(5825):750–4.
Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A 1996;93(20):11213–8.
Brunden KR, Zhang B, Carroll J, Yao Y, Potuzak JS, Hogan AM, et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J Neurosci 2010;30(41):13861–6. doi:10.1523/JNEUROSCI.3059-10.2010.
Bulic B, Pickhardt M, Mandelkow EM, Mandelkow E. Tau protein and tau aggregation inhibitors. Neuropharmacology 2010;59(4–5):276–89. doi:10.1016/j.neuropharm.2010.01.016.
Villemagne VL, Furumoto S, Fodero-Tavoletti MT, Harada R, Mulligan RS, Kudo T, et al. The challenges of tau imaging. Future Neurol 2012;7(4):409–21.
Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007;68(20):1718–25. doi:10.1212/01.wnl.0000261919.22630.ea.
Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med 2006;355(25):2652–63.
Okamura N, Suemoto T, Furumoto S, Suzuki M, Shimadzu H, Akatsu H, et al. Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer’s disease. J Neurosci 2005;25(47):10857–62.
Fodero-Tavoletti MT, Okamura N, Furumoto S, Mulligan RS, Connor AR, McLean CA, et al. 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 2011;134(Pt 4):1089–100. doi:10.1093/brain/awr038.
Zhang W, Arteaga J, Cashion DK, Chen G, Gangadharmath U, Gomez LF, et al. A highly selective and specific PET tracer for imaging of tau pathologies. J Alzheimers Dis 2012;31(3):601–12. doi:10.3233/JAD-2012-120712.
Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis 2013;34(2):457–68. doi:10.3233/JAD-122059.
Shao XM, Carpenter GM, Desmond TJ, Sherman P, Quesada CA, Fawaz M, et al. Evaluation of [11C]N-methyl lansoprazole as a radiopharmaceutical for PET imaging of tau neurofibrillary tangles. ACS Med Chem Lett 2012;3(11):936–41.
Okamura N, Furumoto S, Harada R, Tago T, Yoshikawa T, Fodero-Tavoletti M, et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J Nucl Med 2013;54(8):1420–7. doi:10.2967/jnumed.112.117341.
Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 2013;79(6):1094–108. doi:10.1016/j.neuron.2013.07.037.
Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia C, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis 2014;38:171–84. doi:10.3233/JAD-130098.
Pike VW. PET radiotracers: crossing the blood–brain barrier and surviving metabolism. Trends Pharmacol Sci 2009;30(8):431–40. doi:10.1016/j.tips.2009.05.005.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44.
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 2001;58(11):1803–9.
Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 2007;6(11):1004–14.
Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol 2011;69(1):181–92. doi:10.1002/ana.22248.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25(11):1528–47.
Mukaetova-Ladinska EB, Harrington CR, Roth M, Wischik CM. Biochemical and anatomical redistribution of tau protein in Alzheimer’s disease. Am J Pathol 1993;143(2):565–78.
Khatoon S, Grundke-Iqbal I, Iqbal K. Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett 1994;351(1):80–4.
Larner AJ. The cerebellum in Alzheimer’s disease. Dement Geriatr Cogn Disord 1997;8(4):203–9.
Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008;64(4):388–401.
Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010;31(8):1275–83. doi:10.1016/j.neurobiolaging.2010.04.007.
Delacourte A, Sergeant N, Wattez A, Maurage CA, Lebert F, Pasquier F, et al. Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp Gerontol 2002;37(10–11):1291–6.
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 2013;12(4):357–67. doi:10.1016/S1474-4422(13)70044-9.
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45(3):358–68.
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992;42(3 Pt 1):631–9.
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 1999;46(6):860–6.
Harada R, Okamura N, Furumoto S, Tago T, Maruyama M, Higuchi M, et al. Comparison of the binding characteristics of [18F]THK-523 and other amyloid imaging tracers to Alzheimer’s disease pathology. Eur J Nucl Med Mol Imaging 2013;40(1):125–32. doi:10.1007/s00259-012-2261-2.
Acknowledgements
We thank Prof. Michael Woodward, Dr. John Merory, Dr. Gordon Chan, Dr. Kenneth Young, Dr. David Darby, Ms. Fiona Lamb and the Brain Research Institute for their assistance with this study. The study was partially supported by an Alzheimer Drug Discovery Foundation Research Grant (20101208 AFTD) and by a National Health and Medical Research Council of Australia Project Grant 1044361. The funding sources had no input into the design and conduct of the study; collection, management, analysis and interpretation of the data; and in the preparation, review, approval or decision to submit the manuscript for publication.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ contributions
Dr. Villemagne had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Drs. Villemagne, Furumoto, Fodero-Tavoletti, Kudo, Rowe and Okamura participated in the design, acquisition, analysis and interpretation of the data and writing of this manuscript.
Study concept and design: Villemagne, Okamura, Kudo, Rowe
Acquisition of data: Villemagne, Rowe, Fodero-Tavoletti, Pejoska, Yates, Piguet, Mulligan
Analysis and interpretation of data: Villemagne, Doré, Rowe, Okamura
Drafting of the manuscript: Villemagne, Okamura
Critical revision of the manuscript for important intellectual content: Villemagne, Furumoto, Rowe, Harada, Fodero-Tavoletti, Piguet, Hodges, Yanai, Masters, Kudo, Okamura
Statistical analysis: Villemagne, Okamura
Study supervision: Villemagne, Okamura
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1123 kb)
Rights and permissions
About this article
Cite this article
Villemagne, V.L., Furumoto, S., Fodero-Tavoletti, M.T. et al. In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging 41, 816–826 (2014). https://doi.org/10.1007/s00259-013-2681-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2681-7