Abstract
Purpose
To evaluate the impact of dosimetry based on MAA SPECT/CT for the prediction of response, toxicity and survival, and for treatment planning in patients with hepatocellular carcinoma (HCC) treated with 90Y-loaded glass microspheres (TheraSphere®).
Methods
TheraSphere® was administered to 71 patients with inoperable HCC. MAA SPECT/CT quantitative analysis was used for the calculation of the tumour dose (TD), healthy injected liver dose (HILD), and total injected liver dose. Response was evaluated at 3 months using EASL criteria. Time to progression (TTP) and overall survival (OS) were evaluated using the Kaplan-Meier method. Factors potentially associated with liver toxicity were combined to construct a liver toxicity score (LTS).
Results
The response rate was 78.8 %. Median TD were 342 Gy for responding lesions and 191 Gy for nonresponding lesions (p < 0.001). With a threshold TD of 205 Gy, MAA SPECT/CT predicted response with a sensitivity of 100 % and overall accuracy of 90 %. Based on TD and HILD, 17 patients underwent treatment intensification resulting in a good response rate (76.4 %), without increased grade III liver toxicity. The median TTP and OS were 5.5 months (2–9.5 months) and 11.5 months (2–31 months), respectively, in patients with TD <205 Gy and 13 months (10–16 months) and 23.2 months (17.5–28.5 months), respectively, in those with TD >205 Gy (p = 0.0015 and not significant). Among patients with portal vein thrombosis (PVT) (n = 33), the median TTP and OS were 4.5 months (2–7 months) and 5 months (2–8 months), respectively, in patients with TD <205 Gy and 10 months (6–15.2 months) and 21.5 months (12–28.5 months), respectively, in those with TD >205 Gy (p = 0.039 and 0.005). The median OS was 24.5 months (18–28.5 months) in PVT patients with TD >205 Gy and good PVT targeting on MAA SPECT/CT. The LTS was able to detect severe liver toxicity (n = 6) with a sensitivity of 83 % and overall accuracy of 97 %.
Conclusion
Dosimetry based on MAA SPECT/CT was able to accurately predict response and survival in patients treated with glass microspheres. This method can be used to adapt the injected activity without increasing liver toxicity, thus defining a new concept of boosted selective internal radiation therapy (B-SIRT). This new concept and LTS enable fully personalized treatment planning with glass microspheres to be achieved.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC), a highly prevalent tumour, is the third most common cause of cancer-induced death worldwide with more than 500,000 deaths per year [1]. The clinical effectiveness of selective internal radiation therapy (SIRT) using 131I-radiolabelled lipiodol was first demonstrated in 1994 based on a randomized study in patients with portal vein thrombosis (PVT) [2]. Despite its proven efficacy, 131I-radiolabelled lipiodol has not been widely used due to major radioprotection constraints. The development of 90Y-loaded microspheres is a great advance, and 90Y-loaded microspheres are currently increasingly used in patients with Barcelona Clinic Liver Cancer stage B (intermediate stage) liver cancer and in patients with PVT, with promising clinical outcomes [3–6]. In particular, one retrospective study has shown that glass microspheres are able to achieve a significantly improved rate of down-staging compared to chemoembolization, with significantly fewer side effects [7], while another study has shown that progression-free survival (PFS) is significantly improved [8]. Regarding safety considerations, to identify lung and digestive shunts, SIRT with 90Y-loaded microspheres is preceded by diagnostic angiography and hepatic perfusion scintigraphy with 99mTc macroaggregated albumin (MAA). SIRT is currently recognized as a potential approach to local and regional HCC therapy by various scientific societies and expert groups, including the European Society of Medical Oncology [9].
The concept of SIRT is to deliver a tumoricidal dose to the tumour while sparing healthy liver tissue [10, 11]. To achieve the best efficacy and lowest toxicity, the tumour absorbed dose (TD) and the dose absorbed by healthy liver tissue are two key parameters that need to be known and controlled when using SIRT. From a radiobiological point of view, optimal perfusion and blood flow are prerequisites for SIRT, in contrast to chemoembolization, as oxygen increases radiotoxicity [11]. However, limited data are available concerning tumour dosimetry, and recommendations are still based on liver dosimetry. The goal of therapy with glass microspheres is to deliver a radiation dose of 120 ± 20 Gy to the injected liver volume (ILD), with the dose calculation based on the accepted simplified formula [12]: ILD = IA × (1 − S) × 50/W, where IA is the activity in gigabecquerels to be injected, S is the percentage pulmonary shunt as measured by a MAA liver perfusion scan, and W is the mass in kilograms of the liver volume to be treated.
When using resin microspheres, guidelines relying on expert opinion recommend using the partition model [11]. For delineable tumours, an ILD of 120 Gy to the tumour compartment is recommended and 50–70 Gy to the healthy liver, depending on the clinical scenario. For nondelineable tumours the body surface area method is used.
Preliminary results have shown that there is a close correlation between TD and response with the use of glass microspheres [5, 13, 14]. In light of this, our team has put forward the possibility of using predictive dosimetry based on MAA SPECT/CT in treatment intensification, as we have previously identified a mean tumour threshold dose (TTD) of 205 Gy to achieve a response [14]. The aim of this retrospective study was to determine the impact of MAA SPECT/CT dosimetry on treatment planning and survival when using glass microsphere therapy in a large patient population, and to identify factors predictive of liver toxicity.
Material and methods
Patient characteristics
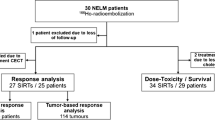
Data from all 86 HCC patients referred to our institution for SIRT with 90Y-loaded glass microsphere (TheraSphere®; Nordion, Ottawa, Canada) in a palliative setting were analysed. After exclusion of 15 patients, 11 following diagnostic angiography (high hepatopulmonary shunting, 4; poor tumour targeting, 6; noncorrectable digestive shunt, 1) and 4 due to lack of SPECT/CT acquisitions, 71 treated patients were included in this retrospective study. Written informed consent was obtained from each patient, and the use of SIRT was approved by the ethics committee of our university hospital. The indication for SIRT was clarified during a HCC multidisciplinary staff meeting. SIRT was used as first-line treatment in 35 patients, and for recurrences in the remaining 36 patients (15 after surgery, 14 after chemoembolization, 5 after treatment with sorafenib, and the remaining 2 after 131I-lipiodol therapy). Patients were considered unsuitable for chemoembolization because of the presence of different conditions (PVT, multifocal or voluminous lesions, incomplete response, or progression after several cycles of chemoembolization). Most patients (33) had intermediate or advanced stage disease with PVT (12 main, lobar, and 21 segmental), but without extrahepatic spread. Patient and tumour characteristics are detailed in Table 1.
Planning and administration of 90Y-loaded glass microspheres
Microspheres were administered as described by Salem et al. [12]. After diagnostic angiography, a liver perfusion scan was performed, following injection of 185 MBq of MAA into the hepatic artery. Planar scans were acquired for lung shunt evaluation. In addition, SPECT/CT scans acquired (window 140 ± 7.5 KeV, 32 projections, 180°, 128 × 128, 30 s per projection; Symbia T2 gantry; Siemens, Germany), and data was reconstructed using an iterative method (OSEM, five iterations, eight subsets) with attenuation (low-dose CT attenuation map) and scatter corrections (Jaszczak method, diffusion window 120 ± 7.5 KeV), and then visualized with and without CT scan data fusion.
As previously described, the “volumetric analysis” software (Syngo workstation; Siemens) was used to quantitatively analyse tumour and nontumour liver tissue uptake [15]. This software enables semiautomatic generation of the volume of interest (VOI) in the injected liver and tumour using an isocontour method. For each VOI, the threshold value was adjusted so that the isocontours of the MAA distribution volume on the fusion images matched the liver and tumour boundaries. Regarding tumour delineation, the model was applied to all tumours visualized by SPECT/CT, including small and infiltrative tumours. These VOIs were then used to measure the MAA volume of distribution in the injected liver and tumour, as well as the total activity contained in the injected liver and tumour. In necrotic tumours, the volume of the hypofixing area was not taken into account by the software (Fig. 1). Volume and activity in the injected healthy liver were calculated by subtraction.
VOI delineation in a 77-year-old patient with a large HCC of the right lobe associated with two small lesions of the left lobe who underwent treatment intensification (boosted). a Initial CT slice shows heterogeneous HCC of 12.3 cm with a central area of necrosis. b, d MAA SPECT/CT with high uptake and a hypofixing area: b VOI delineation for the tumour; d VOI delineation for the injected liver. The patient underwent treatment intensification with 3.5 GBq of 90Y-loaded glass microspheres (ILD 162 Gy, TD 280 Gy, HILD 54 Gy). Using the standard approach, only 2.6 GBq of 90Y-loaded glass microspheres would have been used to achieve an ILD of 120 Gy, then the TD would have been only 207 Gy, and the HILD 38 Gy. c CT slice 3 months after injection shows EASL partial response of the treated tumour of the right lobe. The left lesions were treated by hyperselective chemoembolization due to their small size. The patient was still alive, but with progressive disease, at 28.5 months (most recent follow-up visit)
The activity to be injected was calculated conventionally for the schedule in order to achieve an ILD of 120 ± 20 Gy (without exceeding 30 Gy for the lungs) in the first 20 patients. After the 20th patient, the standard approach was used to verify that the tumour dose was effectively higher than the TTD of 205 Gy. If this threshold was not reached, patients underwent treatment intensification (increasing the activity to be injected, i.e. boosted patients) with the goal of achieving a tumour dose exceeding 205 Gy while maintaining the healthy injected liver dose (HILD) below 120 Gy, as previously described [14]. The glass microspheres were injected using a lobar approach 8 to 15 days later. In patients with bilateral disease, two lobar treatments were administered separately at an interval of 6 to 8 weeks. No specific activity adaptation was made with respect to the bilateral nature of the lesions, nor was any specific activity adaptation made in patients regarding the HILD who were potentially at risk of hepatic toxicity (bilateral disease, Child-Pugh class B).
Response evaluation
Tumour response was assessed using the European Association for the Study of the Liver (EASL) criteria [16]. Triphasic CT scans were performed 3 months after treatment, and then every 3 months until progression or death. For EASL response evaluation, complete response (CR) was defined as absence of any enhancing tissue, partial response (PR) as a decrease of >50 % in enhancing tissue, progressive disease (PD) as an increase of >25 % in the size of one or more measurable lesion(s) or the appearance of new lesions, and stable disease (SD) as a tumour response between PR and PD.
Liver toxicity factors
Only definitive grade III or higher liver toxicity was considered by means of the Common Terminology Criteria for Adverse Events (v4). Imputability of the suspected toxicity was defined according to ICH E2B (R3) guidelines, meaning that toxicity was attributed to disease progression in patients with both liver toxicity and evidence of largely PD. Different parameters were evaluated: presence of PVT, alpha-fetoprotein level, treatment line (first line versus second or further lines), bilirubin level (≤ or >34 μmol/ml), alanine aminotransferase (ALT) level (less than or more than five times normal), HILD (>40, 6, 80, 100 and 120 Gy), Child-Pugh class and score (A or B7), tumour involvement (< or >70 %), hepatic reserve (percentage of nonirradiated liver, < or >30 %), severe underlying biliary disease (biliary cirrhosis or intrahepatic biliary dilatation), presence of complete PVT of the trunk without MAA targeting on SPECT/CT, as well as the combination of HILD (>40, 60, 80, 100, and 120 Gy) and hepatic reserve <30 %.
Liver toxicity scores (LTS) were based on recognized pretreatment factors previously identified as being linked with toxicity as well as factors identified in this study, including ALT level more than five times normal, bilirubin level >35 μmol/ml, tumour involvement >70 %, Child-Pugh class B, previous treatment, severe underlying biliary disease, presence of large PVT of the trunk without MAA targeting on SPECT/CT, and a combined profile of HILD >120 Gy with a hepatic reserve <30 %. Different weightings for each parameter were used to create different LTS (Table 2):
-
LTS 1: one point for each parameter present
-
LTS 2: one point for the first five parameters and two points for the last three
-
LTS 3: one point for the first five parameters and three points for the last three
For each LTS, different cut-off values were evaluated in order to obtain the best score for liver toxicity detection. A patient’s score was considered positive (predictive of liver toxicity) if its value was higher than or equal to a predefined cut-off value.
Statistics
Quantitative values are expressed as means ± standard deviation. The TD in responding and nonresponding patients were compared using Wilcoxon’s distribution-free comparison test. Regarding the responses, a true-positive (TP) response was defined as an objective response (CR + PR) obtained when the TD was higher or equal to the TTD. A true-negative (TN) response was defined as TD < TTD, without an objective response at 3 months. A false-negative (FN) response was defined as TD < TTD, with an objective response at 3 months. A false-positive (FP) response was defined as TD ≥ TTD, without an objective response at 3 months.
Univariate analysis and Wilcoxon’s distribution-free comparison test were used to identify parameters associated with tumour response, PFS, and overall survival (OS). Tested parameters for response were: injected activity, ILD (gray), HILD (gray), TD (gray), tumour to nontumour ratio, tumour activity (percent injected activity), alpha-fetoprotein level (kIU/l), and tumour size (cm). The tested parameters for survival were: tumour type (unifocal versus multifocal and infiltrative), size (≤5 versus >5 cm), PVT (present), alpha-fetoprotein level (≤400 versus >400 kIU/l), Child-Pugh class (A versus B7), CLIP (stage 0, 1 or 2 versus 3 or 4) and TD (< versus ≥205 Gy). Data selected after univariate analysis was then subjected to multivariate analysis using logistic regression testing. Time to progression (TTP) was defined as the time between treatment and disease progression confirmed on CT scan. OS was defined as the time between treatment and the most recent follow-up or death. Survival rates were estimated using the Kaplan-Meier method, and they were compared using the log-rank test.
For liver toxicity, univariate analysis was carried out using Fisher’s exact test, and multivariate analysis using a logistic regression. Liver toxicity scoring systems were compared using ROC analysis. Response rates and toxicity were compared using the chi-squared test. Finally the LTS were compared with ROC curves. SAS software was employed for the statistical analyses, with a significance threshold set at p ≤ 0.05.
Results
In total, 93 injections were administered to 71 patients, of whom 22 received two treatments (8 for bilateral lesions, 2 for incomplete injection of the initial, 12 for incomplete response or recurrence after the first injection). For the overall population, the mean 90Y-loaded glass microsphere injected activity was 2.9 ± 1.5 GBq, the mean ILD was 124 ± 35 Gy, and the mean HILD was 76 ± 35 Gy.
Response evaluation
The 3-month response rate was 78.8 %, comprising 7 patients with CR, 49 with PR, 11 with SD and 4 with PD. The response rate was 55 % in the first 20 patients treated without intensification intent and 86 % in the following 51 patients treated with intensification intent (p = 0.001). Based on 99mTc-MAA SPECT/CT, TD was 342 ± 116 Gy for responding lesions, and 191 ± 89 Gy for nonresponding lesions (p < 0.001). Considering a TTD of 205 Gy, MAA SPECT/CT predicted response with a sensitivity of 100 % and overall accuracy of 90 % (no FN, seven FP). Eight patients with a TD <205 Gy did not respond to treatment, while 56 out of 63 patients with a TD >205 Gy did respond. Of the seven patients with a FP response, four had a TD <275 Gy (between 205 and 275 Gy), which corresponds to a FP rate of 15.4 %. The three remaining patients with a FP response had a TD >275 Gy corresponding to a FP rate of 8.1 % when taking into account only patients receiving a TD >275 Gy. Finally, the FP rate was 0 % in patients with TD >350 Gy.
The response rate was 81 % in the 33 patients with PVT (3 with CR, 24 with PR, 4 with SD and 2 with PD), of whom nine had partial or total portal vein recanalization (Fig. 2). Five patients with PVT (i.e. 15.6 %) were accurately downstaged, and two of these underwent treatment intensification. One of these five patients refused transplantation, and four underwent lobar hepatectomy.
A 62-year-old patient with a large HCC and main PVT who underwent treatment intensification (boosted). The patient showed a major response with revascularization of the portal vein. a–c Initial imaging: CT slices show a heterogeneous HCC of 9.6 cm (a) with main PVT (b); MAA SPECT/CT image shows high uptake in the main PVT (c) The patient underwent treatment intensification with 1.16 GBq of 90Y-loaded glass microspheres (ILD 211 Gy, TD 285 Gy, HILD 65 Gy). Using the standard approach only 0.56 GBq of 90Y-loaded glass microspheres would have been used to achieve an ILD of 120 Gy, then the TD would have been only 162 Gy and the HILD 37 Gy. d, e CT slices 3 months after injection show EASL partial response of the tumour (d) and main portal vein revascularization (e). The patient subsequently received a left hepatectomy (with complete tumour resection). PFS was 15 months with lung recurrence only. The patient was still alive at the most recent follow-up visit (18 months)
MAA uptake in PVT on SPECT/CT was observed in 25 of the 26 responding patients (Fig. 2), but only in one of the six nonresponding patients who had a very large tumour resulting in a low tumour dose. In total, 12 patients had main PVT. Eight of these showed good MAA targeting, of whom seven were responders, and tolerated the treatment well, and one did not responding but had a low TD. The remaining four showed no MAA uptake, and all four had severe liver toxicity, which was transient in one, and definitive in the other three.
In the 21 patients with segmental or lobar PVT, the response rate was 90.5 %. In the univariate analysis, only the tumour to nontumour ratio and the TD proved to be significantly correlated with response (p = 0.01 and 0.001, respectively). In the multivariate analysis, TD was still correlated with response (p = 0.019).
Treatment intensification
Overall, 17 patients received a boost, i.e. 24 % of the whole population (n = 71), but after identification of the concept and the TTD (after the 20th patient) the intensification rate was 33 % (17/51 patients). The intensification rate was only 5 % in patients with lesions <5 cm versus 50 % in those with lesions ≥5 cm. In patients with PVT, the intensification rate was 38 %. Only two patients with bilateral lesions underwent intensification, which was performed only for the first treatment. The second lobe was treated 6 to 8 weeks later using the standard dosimetric approach, without complications. Intensification parameters are presented in Table 3.
Survival analysis
The median TTP was 11 months (95 % CI 8–15 months) and the median OS was 23 months (95 % CI 15.3–28 months). In patients with TD <205 Gy, the median TTP was 5.5 months (95 % CI 2–9.5 months) versus 13 months (95 % CI 10–16 months) in those with TD >205 Gy (p = 0.0038). In patients with TD <205 Gy, the median OS was 11.5 months (95 % CI 2–30.7 months) versus 23.2 months (95 % CI 17.5–28.5 months) in those with TD >205 Gy (not significant; Fig. 3).
In patients with PVT, the median TTP was 10 months (95 % CI 6–15 months) and the median OS was 18.2 months (95 % CI 11–27 months). In patients with PVT and TD <205 Gy, the median TTP was 4.5 months (95 % CI 2–7 months) versus 10 months (95 % CI 6–15.2 months) in those with TD >205 Gy (p = 0.048), while in patients with PVT and TD <205 Gy, the median OS was 5 months (95 % CI 2–8 months) versus 21.5 months (95 % CI 18–28.5 months) in those with TD >205 Gy (p = 0.005; Fig. 4).
In patients with PVT, TD <205 Gy and/or no MAA targeting of the PVT (n = 5), the median TTP and OS were 2 months (95 % CI 2–7 months) and 3 months (95 % CI 2–8 months), respectively, versus 13 months (95 % CI 6–15 months; p = 0.0009) and 24.5 months (95 % CI 18–28.5 months; p < 0.0001), respectively, in patients with PVT, TD >205 Gy and good PVT targeting (n = 28, i.e. 85 % of patients with PVT; Fig. 5).
Lastly, in patients with segmental or lobar PVT, all of whom exhibited a TD higher than 205 Gy, the median TTP and OS were 10 months (95 % CI 6–18 months) and 24.50 months (95 % CI 12–28.7 months), respectively. In Child-Pugh class A patients (n = 18), the median TTP and OS were 13 months (95 % CI 7–18 months) and 24.5 months (95 % CI 11 months to infinity), respectively (Fig. 6), versus 6 months (95 % CI 6–10 months) and 18 months (95 % CI 17.5–27 months) in Child-Pugh class B patients (n = 3; not significant).
The only parameters that were correlated with PFS in the univariate analysis were alpha-fetoprotein level and TD (p = 0.009 and 0.0002, respectively). Notably, PVT was shown not to be significantly correlated with PFS. In the multivariate analysis, only TD was still significantly associated with PFS (p < 0.0001). The univariate analysis revealed that TD was the only parameter that was correlated with OS (p = 0.04), whereas no parameter was significantly correlated with OS in the multivariate analysis.
Hepatic toxicity and global tolerance
No deaths during the first month of treatment were reported. Six patients developed permanent grade 3 or higher hepatic toxicity beginning during the month following treatment without evidence of progression. The toxicity rate was not significantly different between the patients without treatment intensification (9.2 %, 5/54) and those who underwent treatment intensification (5.8 %, 1/17; not significant). One patient with biliary cirrhosis, who had a combination of HILD >120 Gy and hepatic reserve <30 %, died of liver failure at 2 months. Three patients with deep main PVT without MAA uptake died of liver failure at 3, 3 and 3.5 months, respectively. One patient with well-compensated cirrhosis (Child-Pugh class A5) and a relatively small tumour had unexplained refractory ascites and died at 7 months of ascites infection. The remaining patient developed grade 3 ascites following treatment and died at 14 months of hepatic failure without evidence of disease progression. He had a combination of HILD >120 Gy and hepatic reserve <30 %. Only one of these patients exhibiting complete main PVT without MAA uptake had undergone treatment intensification, while the five others received standard doses (120 ± Gy to the injected liver).
In the univariate analysis, only severe biliary disease (p = 0.006), large main PVT without MAA uptake (p = 0.0014), a combination of HILD >100 Gy and hepatic reserve <30 % (p = 0.032), and combination of HILD >120 Gy and hepatic reserve <30 % (p = 0.017) were significantly associated with a risk of liver toxicity, as shown in Table 4. In the multivariate analysis, severe biliary disease, main PVT without MAA uptake and combination of HILD >120 Gy and hepatic reserve <30 % were still significantly associated with liver toxicity (Table 4).
LTS 3 with a cut-off value of 3 points showed the best diagnostic performance (Fig. 7). It was able to predict severe permanent liver toxicity with a sensitivity of 83 %, a negative predictive value of 98 %, a positive predictive value of 71 %, and an overall accuracy of 97 %.
Furthermore, discrete to moderate asthenia was frequently reported, whereas episodes of pain were less commonly noted. One patient presented with septicaemia, probably caused by diagnostic angiography. No other severe extrahepatic complications were observed.
Discussion
There are five important results of this study: confirmation of the accuracy of MAA SPECT/CT dosimetry in predicting response, development of the intensification concept, good results in patients with PVT, identification of an accurate toxicity scoring system, and finally availability of a fully personalized approach. Indeed, our study confirmed previous preliminary results reported by Mazzaferro et al. [5], Chiesa et al. [13] and our team [14], notably the high correlation between TD and response using glass microspheres, along with a TTD necessary to achieve a response. This point is evident from a radiobiological point of view, as radioinduced damage responds to an “all or nothing” concept, with no damage occurring below the TTD and lethal damage occurring above the TTD (the higher the TD above the TTD, the greater the damage). In the current study, the TTD of 205 Gy showed good diagnostic performance in selecting responders prior to treatment initiation, with a sensitivity of 100 % and an accuracy of 90 %. We observed a FP response prediction in seven patients (i.e. patients with TD >205 Gy and no response). These were patients with large lesions (mean size 9.2 ± 3.4 cm), with a large area of tumour necrosis seen in three of them prior to treatment, and thus presenting a higher risk of hypoxia-induced radioresistance.
Even if the TTD identified in the three studies (5, 13, 14) were different, probably due to different dosimetric approaches (MIRD approach and mean TD in one study, BED approach and voxel TD in the other), its identification has a major impact. To date, no factors predicting response or survival other than TD have been identified, and even the recent study conducted by Sato et al. failed to establish a correlation between lesion vascularization and survival [17]. The fact that a TTD has been identified by two independent teams using different dosimetric approaches underlines the robustness of dosimetry based on MAA SPECT/CT with glass microspheres.
Given that MAA SPECT/CT dosimetry is available prior to the injection of microspheres, treatment intensification can be proposed for patients with a TD just below or close to the TTD, which is in accordance with the new concept of boosted SIRT (B-SIRT). In our study, we applied this new concept to 17 patients, with a satisfactory clinical outcome: the response rate in these difficult patients with large lesions was 76 %, and the severe liver toxicity rate was only 5.8 %, versus 9.2 % in nonboosted patients. As the FP rate for response detection was lower in patients receiving a TD >275 Gy compared to those receiving a lower TD (i.e. 8.1 % versus 15.4 %) and as there were no patients with a FP response among those who received a TD ≥350 Gy, treatment intensification to obtain a TD above the TTD of 205 Gy and could even exceed 275 Gy or eventually 350 Gy may be possible.
It is probably preferable to optimize the activity rather than perform several treatments in succession such as in fractionated external beam radiotherapy as proposed by several other teams [18]. Previously, we have demonstrated using 131I-lipiodol that the TD is more effective following initial treatment (248 ± 176 Gy) rather than after the second treatment (152 ± 122 Gy), which suggests that the intratumour accumulation of the therapeutic agent decreases under the effect of previous treatments due to redistribution from tumour tissue into nontumour liver tissue [19].
It should be noted that the concept of intensification appears to be of major interest in patients with PVT (38 % of patients) and for those with lesions ≥5 cm (50 % of patients). This point is of particular interest, as a complete histological response has been reported following glass microsphere administration in only 33 % of tumours exceeding 5 cm [20]. Therefore, the concept of treatment intensification could be associated with a significantly increased efficacy of glass microspheres for lesions larger than 5 cm. Our clinical results were particularly interesting in patients PVT. The OS achieved, 18.2 months and 24.5 months for Child-Pugh class A patients with segmental or lobar PVT, compared favourably with that reported by other teams using glass microspheres (between 10 and 13 months [3–5]) or resin microspheres (10.0 months [6]), and even sorafenib (8.1 months [21]). These good results may be accounted for by this new concept of treatment intensification. Nonetheless, our positive results must be confirmed in a randomized study.
Another major finding of our study was the identification of a relationship between TD and survival. Survival can be predicted using a TTD of 205 Gy. When the dose delivered to the tumour was equal to or higher than this threshold value, median TTP increased from 5.5 to 13 months (p = 0.0038), and OS from 11.5 to 23.2 months. The results were even more convincing in patients with PVT with an OS of only 5 months for a TD <205 Gy and 21.5 months for a TD >205 Gy (p = 0.005). These results (good predictive value for response and survival of MAA dosimetry) seem less reproducible when using resin microspheres. The two products differ, as resin microspheres have a more embolic effect, and their radiobiological effects are probably slightly different [22]. Published results regarding MAA scintigraphy and dosimetry are discordant. Using planar scintigraphy and the partition model, Ho et al. [23] reported a response rate in HCC of 37.5 % for a TD >225 Gy versus only 10.3 % for a TD ≤225 Gy (p < 0.006). However, two recent studies have shown an overall poor correlation between the uptakes of MAA and 90Y-resin microspheres [24, 25], with a good correlation found in some patients and a very poor correlation in others [24]. Further work on resin microspheres dosimetry is still required, and the concept of treatment intensification based on MAA SPECT/CT dosimetry should also be evaluated for resin microspheres, as it may be a valuable approach in a patient subpopulation that still needs to be identified.
Liver tolerance was acceptable with permanent severe liver function alterations observed in only six patients (i.e. 8.4 %), and was not increased in patients who underwent treatment intensification. Several parameters have been reported previously by different teams to be associated with a high risk of liver toxicity, including ALT more than five times normal [26], bilirubin level >34 μmol/ml [26], tumour involvement >70 % [26], Child-Pugh class B [3, 5, 26] as well as previous treatment [27]. In our study, these parameters were not significantly associated with severe liver toxicity, which is probably because of our careful patient selection, with only two patients with ALT levels more than five times normal, two with bilirubin levels >2 mg, and two with tumour involvement >70 %. In the univariate analysis, severe underlying biliary disease (one patient with biliary cirrhosis and another with diffuse biliary dilatation) was identified as being correlated with liver toxicity. In the multivariate analysis, two other original and robust (i.e. significant) prognostic factors for liver toxicity were identified, namely large main PVT without MAA targeting and combination of HILD >120 Gy and hepatic reserve <30 %. It should be noted that, in our study, the HILD alone or the hepatic reserve alone was not associated with a risk of liver toxicity. Using glass microspheres, Chiesa et al. reported a higher risk of liver toxicity (but taking into account grade 2 toxicity) with a mean lobe dose exceeding 90 Gy for patients with Child-Pugh class >A5 (i.e. 80 % versus 50 % with a mean lobe dose <90 Gy) [13]. Using resin microspheres, liver toxicity of grade 3 or more was reported in 21 % of patients with a liver median dose of only 36 Gy [28].
The LTS identified in our study, integrating two major MAA SPECT/CT parameters, was able to detect the risk of severe liver toxicity with a sensitivity of 83 %, a negative predictive value of 98 %, a positive predictive value of 71 %, and an overall accuracy of 97 %. To our knowledge, a similar liver toxicity scoring system has not previously been described. Severe permanent liver toxicity was missed in only one patient using the scoring system. As the patient showed no recognized risk factors indicating liver toxicity, the liver toxicity in this patient was possibly related to constitutional radiation hypersensitivity. This LTS needs to be improved, and its prognostic value confirmed prospectively in a larger, and perhaps a less carefully selected patient population than that in our study.
Finally, SIRT with glass microspheres is a fully personalized approach, which is the standard endpoint to aim for in oncology [29, 30]. Using MAA SPECT/CT dosimetry, adaptation of the injected activity in relation to the TD is possible, and some patients may undergo treatment intensification in order to achieve a better response and improved survival. On the other hand, the toxicity scoring system has proven helpful in the identification of patients at high risk of severe permanent liver toxicity, and can be used for excluding such patients from treatment. For example, when using the B-SIRT concept in patients with PVT, MAA SPECT/CT is able to identify, before therapy, a group of very poor candidates (TD <205 Gy and/or no MAA targeting of PVT) with an OS of only 3 months, and a group of good candidates, representing 85 % of patients with PVT, with an OS of 24.5 months. Such predictive factors and high OS have not been reported previously for patients with PVT regardless of the therapeutic approach used.
Concerning the technical aspects, the dosimetric model used in this study is very simple, and is only able to provide an evaluation of the mean dose of the hypervascularized lesions (in necrotic lesions, the software does not take into account the volume of necrosis for definition of the VOI). A mean dose evaluation may be insufficient for heterogeneous lesions, and for the evaluation of HILD as well, as it does not take into account heterogeneity of the irradiation and irradiation via the tumour of the healthy peritumoral liver, an effect that could prove significant for large tumours with enhanced uptake. A voxel-based dosimetric model [5, 14, 31] may lead to progress in this field.
Conclusion
This study involving a large patient cohort demonstrated the predictive value of tumour dosimetry based on MAA SPECT/CT using glass microspheres in terms of response to SIRT, PFS, and OS. Tumour dosimetry data from MAA SPECT/CT enables the clinical use of treatment intensification or B-SIRT, with favourable clinical outcomes and low rates of severe liver toxicity, especially in patients with tumours larger than 5 cm and in patients with PVT. B-SIRT is most likely a crucial concept for increasing the effectiveness of SIRT in large lesions using glass microspheres. The new LTS presented in this here allows accurate prediction of severe permanent liver toxicity, thereby facilitating the selection of appropriate candidates. Both the glass microsphere B-SIRT concept and the LTS enable fully customized treatment planning to be achieved, which is the aim in oncology. In the near future, randomized studies are needed to confirm these encouraging results and to better define the role of glass microsphere B-SIRT in the therapeutic management of HCC patients with or without PVT.
References
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50.
Raoul JL, Guyader D, Bretagne JF, Duvauferrier R, Bourguet P, Bekhechi D, et al. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131-iodized oil versus medical support. J Nucl Med. 1994;35:1782–7.
Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64.
Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52(5):1741–9.
Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium(90) radioembolization for intermediate-advanced hepatocarcinoma: A phase II study. Hepatology. 2012. doi:10.1002/hep.26014.
Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al.; European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54(3):868–78.
Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9(8):1920–8.
Memon K, Kulik L, Lewandowski RJ, Wang E, Riaz A, Ryu RK, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology. 2011;141(2):526–35, 535.e1-2.
Jelic S, Sotiropoulos GC; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(5):v59–64.
Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56(2):464–73.
Lau WY, Lai EC, Leung TW. Current role of selective internal irradiation with yttrium-90 microspheres in the management of hepatocellular carcinoma: a systematic review. Int J Radiat Oncol Biol Phys. 2011;81(2):460–7.
Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al.; Technology Assessment Committee; Interventional Oncology Task Force of the Society of Interventional Radiology. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22(3):265–78.
Chiesa C, Maccauro M, Romito R, Spreafico C, Pellizzari S, Negri A, et al. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with 90Y microspheres: the experience of the National Tumor Institute of Milan. Q J Nucl Med Mol Imaging. 2011;55(2):168–97.
Garin E, Lenoir L, Rolland Y, Edeline J, Mesba H, Laffont S, et al. 99mTc-MAA SPECT/CT based dosimetry accurately predicts tumour response and survival in HCC patients treated with 90Y-loaded glass microspheres: preliminary results. J Nucl Med. 2012;53(2):255–63.
Garin E, Rolland Y, Lenoir L, Pracht M, Mesbah H, Porée P, et al. Utility of quantitative Tc-MAA SPECT/CT for yttrium-labelled microsphere treatment planning: calculating vascularized hepatic volume and dosimetric approach. Int J Mol Imaging. 2011;2011:398051.
Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115(3):616–23.
Sato KT, Omary RA, Takehana C, Ibrahim S, Lewandowski RJ, Ryu RK, et al. The role of tumor vascularity in predicting survival after yttrium-90 radioembolization for liver metastases. J Vasc Interv Radiol. 2009;20(12):1564–9.
Cremonesi M, Ferrari M, Bartolomei M, Orsi F, Bonomo G, Aricò D, et al. Radioembolisation with 90Y-microspheres: dosimetric and radiobiological investigation for multi-cycle treatment. Eur J Nucl Med Mol Imaging. 2008;35(11):2088–96.
Becker S, Laffont S, Rolland Y, Lecloirec J, Boucher E, Raoul JL, et al. Dosimetric evaluation and therapeutic response to vectorized internal radiation therapy of hepatocarcinomas using iodine-131 labelled lipiodol. Nucl Med Commun. 2008;29(9):815–25.
Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Giakoumis Spear G, Mulcahy MF, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49(4):1185–93.
Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57(4):821–9.
Garin E, Rolland Y, Boucher E. Pre-therapeutic dosimetric evaluation and selective internal radiation therapy of hepatocellular carcinoma using yttrium-90-loaded microspheres. J Hepatol. 2013. doi:10.1016/j.jhep.2012.11.050.
Ho S, Lau WY, Leung TWT, Chan M, Johnson PJ, Li AKC. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med. 1997;24:293–8.
Knesaurek K, Machac J, Muzinic M, DaCosta M, Zhang Z, Heiba S. Quantitative comparison of yttrium-90 (90Y)-microspheres and technetium-99m (99mTc)-macroaggregated albumin SPECT images for planning 90Y therapy of liver cancer. Technol Cancer Res Treat. 2010;9(3):253–62.
Knesaurek K, Machac J, Heiba S, Cacinovic C, Zhang Z. Can correlation between Y-90 and Tc-99m MAA SPECT/CT image predict clinical outcome in hepatic Y-90 microsphere therapy. Eur J Nucl Med Mol Imaging. 2010;37(2):S293. Abstract.
Goin JE, Salem R, Carr BI, Dancey JE, Soulen MC, Geschwind JF, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: a risk-stratification analysis. J Vasc Interv Radiol. 2005;16(2 Pt 1):195–203.
Kennedy AS, McNeillie P, Dezarn WA, Nutting C, Sangro B, Wertman D, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74(5):1494–500.
Strigari L, Sciuto R, Rea S, Carpanese L, Pizzi G, Soriani A, et al. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: radiobiologic considerations. J Nucl Med. 2010;51(9):1377–85.
Awada A, Vandone AM, Aftimos P. Personalized management of patients with solid cancers: moving from patient characteristics to tumor biology. Curr Opin Oncol. 2012;24(3):297–304.
Hodgson DR, Wellings R, Harbron C. Practical perspectives of personalized healthcare in oncology. N Biotechnol. 2012;29(6):656–64.
Dieudonné A, Garin E, Laffont S, Rolland Y, Lebtahi R, Leguludec D, et al. Clinical feasibility of fast 3-dimensionnal dosimetry of the liver for treatment planning of hepatocellular carcinoma with 90Y-microspheres. J Nucl Med. 2011;52(12):1930–7.
Conflicts of interest
E. Garin is a consultant to Nordion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Garin, E., Lenoir, L., Edeline, J. et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: a new personalized promising concept. Eur J Nucl Med Mol Imaging 40, 1057–1068 (2013). https://doi.org/10.1007/s00259-013-2395-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2395-x