Abstract
Purpose
[18F]Fluciclovine (anti-[18F]FACBC) is a synthetic amino acid developed for PET assessment of the anabolic component of tumour metabolism in clinical routine. This phase 1 trial evaluated the safety, tracer stability and uptake kinetics of [18F]fluciclovine in patients.
Methods
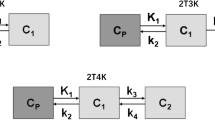
Six patients with biopsy-proven prostate cancer were investigated with 3-T MRI and PET/CT. All underwent dynamic [18F]fluciclovine PET/CT of the pelvic area for up to 120 min after injection of 418 ± 10 MBq of tracer with simultaneous blood sampling of radioactivity. The kinetics of uptake in tumours and normal tissues were evaluated using standardized uptake values (SUVs) and compartmental modelling.
Results
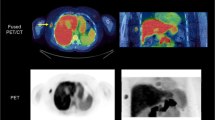
Tumour deposits as defined by MRI were clearly visualized by PET. Urine excretion was minimal and normal tissue background was low. Uptake of [18F]fluciclovine in tumour from the blood was rapid and the tumour-to-normal tissue contrast was highest between 1 and 15 min after injection with a 65 % reduction in mean tumour uptake at 90 min after injection. A one-compartment model fitted the tracer kinetics well. Early SUVs correlated well with both the influx rate constant (K 1) and the volume of distribution of the tracer (V T). There were no signs of tracer metabolite formation. The product was well tolerated in all patients without significant adverse events.
Conclusion
[18F]Fluciclovine shows high uptake in prostate cancer deposits and appears safe for use in humans. The production is robust and the formulation stable in vivo. An early imaging window seems to provide the best visual results. SUV measurements capture most of the kinetic information that can be obtained from more advanced models, potentially simplifying quantification in future studies.





Similar content being viewed by others
References
National Cancer Institute. Surveillance Epidemiology and End Results Program. Cancer of the Prostate Statistics. 2010.
Black RJ, Bray F, Ferlay J, Parkin DM. Cancer incidence and mortality in the European Union: cancer registry data and estimates of national incidence for 1990. Eur J Cancer. 1997;33:1075–107.
Engelbrecht MR, Barentsz JO, Jager GJ, van der Graaf M, Heerschap A, Sedelaar JP, et al. Prostate cancer staging using imaging. BJU Int. 2000;86 Suppl 1:123–34.
Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49:2031–41.
Jadvar H. Molecular imaging of prostate cancer with 18F-fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317–23. doi:10.1038/nrurol.2009.81.
Beheshti M, Imamovic L, Broinger G, Vali R, Waldenberger P, Stoiber F, et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010;254:925–33. doi:10.1148/radiol.09090413.
Picchio M, Briganti A, Fanti S, Heidenreich A, Krause BJ, Messa C, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol. 2011;59:51–60. doi:10.1016/j.eururo.2010.09.004.
Sandblom G, Sörensen J, Lundin N, Häggman M, Malmström P-U. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology. 2006;67:996–1000.
Wachter S, Tomek S, Kurtaran A, Wachter-Gerstner N, Djavan B, Becherer A, et al. 11C-acetate positron emission tomography imaging and image fusion with computed tomography and magnetic resonance imaging in patients with recurrent prostate cancer. J Clin Oncol. 2006;24:2513–9. doi:10.1200/JCO.2005.03.5279.
Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–97.
Seppälä J, Seppänen M, Arponen E, Lindholm P, Minn H. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol. 2009;93:234–40.
Jani AB, Fox TH, Whitaker D, Schuster DM. Case study of anti-1-amino-3-F-18 fluorocyclobutane-1-carboxylic acid (anti-[F-18] FACBC) to guide prostate cancer radiotherapy target design. Clin Nucl Med. 2009;34:279–84.
Yu EY, Muzi M, Hackenbracht JA, Rezvani BB, Link JM, Montgomery RB, et al. C11-acetate and F-18FDG PET for men with prostate cancer bone metastases: relative findings and response to therapy. Clin Nucl Med. 2011;36:192–8. doi:10.1097/RLU.0b013e318208f140.
Pinkawa M, Holy R, Piroth MD, Klotz J, Nussen S, Krohn T, et al. Intensity-modulated radiotherapy for prostate cancer implementing molecular imaging with 18F-choline PET-CT to define a simultaneous integrated boost. Strahlenther Onkol. 2010;186:600–6. doi:10.1007/s00066-010-2122-5.
Meirelles GS, Schoder H, Ravizzini GC, Gonen M, Fox JJ, Humm J, et al. Prognostic value of baseline [18F] fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing metastatic prostate cancer. Clin Cancer Res. 2010;16:6093–9. doi:10.1158/1078-0432.CCR-10-1357.
Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63.
Nye JA, Schuster DM, Yu W, Camp VM, Goodman MM, Votaw JR. Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med. 2007;48:1017–20.
Asano Y, Inoue Y, Ikeda Y, Kikuchi K, Hara T, Taguchi C, et al. Phase I clinical study of NMK36: a new PET tracer with the synthetic amino acid analogue anti-[18F]FACBC. Ann Nucl Med. 2011;25:414–8. doi:10.1007/s12149-011-0477-z.
Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. l-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008;99:2380–6. doi:10.1111/j.1349-7006.2008.00969.x.
McParland BJ, Lax M, Axelsson J, Wall A, Johansson S, Sorensen J. The biodistribution and internal radiation dosimetry of [18F]GE-148 in healthy adult volunteers. Eur J Nucl Med Mol Imaging. 2010;37 (Suppl 2):S287
McConathy J, Voll RJ, Yu W, Crowe RJ, Goodman MM. Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Appl Radiat Isot. 2003;58:657–66.
Kessler RM, Ellis Jr JR, Eden M. Analysis of emission tomographic scan data: limitations imposed by resolution and background. J Comput Assist Tomogr. 1984;8:514–22.
Carson R. Mathematical modeling and compartmental analysis. In: Harbert J, Eckelman WC, Neumann R, editors. Nuclear medicine: diagnosis and therapy. New York: Thieme Medical Publishers; 1996. p. 167–94.
Meyer E. Simultaneous correction for tracer arrival delay and dispersion in CBF measurements by the H215O autoradiographic method and dynamic PET. J Nucl Med. 1989;30:1069–78.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–23. doi:10.1109/TAC.1974.1100705.
Schiepers C, Hoh CK, Nuyts J, Seltzer M, Wu C, Huang SC, et al. 1-11C-acetate kinetics of prostate cancer. J Nucl Med. 2008;49:206–15. doi:10.2967/jnumed.107.044453.
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12.
Higgins KA, Hoang JK, Roach MC, Chino J, Yoo DS, Turkington TG, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUV(mean) has superior prognostic value. Int J Radiat Oncol Biol Phys. 2012;82:548–53. doi:10.1016/j.ijrobp.2010.11.050.
Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE, Patz EF. Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol. 2008;26:1459–64. doi:10.1200/jco.2007.14.3628.
Acknowledgments
We thank Drs. Torsten Danfors, Anders Wall, Gunnar Antoni and Jan Axelsson, and the staff of the PET Center, Uppsala University Hospital, for their support in the study. The trial was sponsored by GE Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sörensen, J., Owenius, R., Lax, M. et al. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur J Nucl Med Mol Imaging 40, 394–402 (2013). https://doi.org/10.1007/s00259-012-2291-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2291-9