Abstract
Purpose
The purpose of this study was to evaluate DNA double-strand breaks (DSBs) in blood lymphocytes of patients undergoing positron emission tomography (PET)/CT using γ-H2AX immunofluorescence microscopy and to differentiate between 18F-fluorodeoxyglucose (FDG) and CT-induced DNA lesions.
Methods
This study was approved by the local Ethics Committee and complies with Health Insurance Portability and Accountability Act (HIPAA) requirements. After written informed consent was obtained, 33 patients underwent whole-body 18F-FDG PET/CT (3 MBq/kg body weight, 170/100 reference mAs at 120 kV). The FDG PET and CT portions were performed as an initial CT immediately followed by the PET. Blood samples were obtained before, at various time points following 18F-FDG application and up to 24 h after the CT scan. Distinct foci representing DSBs were quantified in isolated lymphocytes using fluorescence microscopy after staining against the phosphorylated histone variant γ-H2AX.
Results
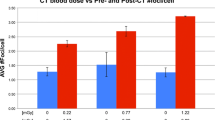
The DSB values at the various time points were significantly different (p < 0.001). The median baseline level was 0.08/cell (range 0.06–0.12/cell). Peaks of radiation-induced DSBs were found 30 min after 18F-FDG administration (median excess foci 0.11/cell, range 0.06–0.27/cell) and 5 min after CT (median excess foci 0.17/cell, range 0.05–0.54/cell). A significant correlation between CT-induced DSBs and dose length product was obtained (ρ = 0.898, p < 0.001). After 24 h DSB values were still slightly but significantly elevated (median foci 0.11/cell, range 0.10–0.14/cell, p = 0.003) compared to pre-exposure levels.
Conclusion
PET/CT-induced DSBs can be monitored using γ-H2AX immunofluorescence microscopy. Peak values may be obtained 30 min after 18F-FDG injection and 5 min after CT. The radionuclide contributes considerably to the total DSB induction in this setting.






Similar content being viewed by others
References
Glaudemans AW, Signore A. FDG-PET/CT in infections: the imaging method of choice? Eur J Nucl Med Mol Imaging 2010;37:1986–91. doi:10.1007/s00259-010-1587-x.
Kwee TC, Basu S, Cheng G, Alavi A. FDG PET/CT in carcinoma of unknown primary. Eur J Nucl Med Mol Imaging 2010;37:635–44. doi:10.1007/s00259-009-1295-6.
Townsend DW. Dual-modality imaging: combining anatomy and function. J Nucl Med 2008;49:938–55. doi:10.2967/jnumed.108.051276.
Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology 2009;251:166–74. doi:10.1148/radiol.2511081300.
Khamwan K, Krisanachinda A, Pasawang P. The determination of patient dose from (18)F-FDG PET/CT examination. Radiat Prot Dosimetry 2010;141:50–5. doi:10.1093/rpd/ncq140.
Brix G, Lechel U, Glatting G, Ziegler SI, Münzing W, Müller SP, et al. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med 2005;46:608–13.
Radiation dose to patients from radiopharmaceuticals. A report of a Task Group of Committee 2 of the International Commission on Radiological Protection. Ann ICRP 1987;18:1–377.
Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med 2002;43:210–4.
Valentin DJ. 3. Recalculated dose data for 19 frequently used radiopharmaceuticals from ICRP Publication 53. Ann ICRP 1998;28:47–83.
Löbrich M, Rief N, Kühne M, Heckmann M, Fleckenstein J, Rübe C, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A 2005;102:8984–9. doi:10.1073/pnas.0501895102.
Kuefner MA, Hinkmann FM, Alibek S, Azoulay S, Anders K, Kalender WA, et al. Reduction of X-ray induced DNA double-strand breaks in blood lymphocytes during coronary CT angiography using high-pitch spiral data acquisition with prospective ECG-triggering. Invest Radiol 2010;45:182–7. doi:10.1097/RLI.0b013e3181d3eddf.
Kuefner MA, Grudzenski S, Schwab SA, Azoulay S, Heckmann M, Heinrich MC, et al. X-ray-induced DNA double-strand breaks after angiographic examinations of different anatomic regions. Rofo 2009;181:374–80. doi:10.1055/s-0028-1109063.
Kuefner MA, Grudzenski S, Schwab SA, Wiederseiner M, Heckmann M, Bautz W, et al. DNA double-strand breaks and their repair in blood lymphocytes of patients undergoing angiographic procedures. Invest Radiol 2009;44:440–6. doi:10.1097/RLI.0b013e3181a654a5.
Antonelli F, Belli M, Cuttone G, Dini V, Esposito G, Simone G, et al. Induction and repair of DNA double-strand breaks in human cells: dephosphorylation of histone H2AX and its inhibition by calyculin A. Radiat Res 2005;164:514–7.
Silbernagl S, Despopoulos A. Color atlas of physiology. 6th ed. New York: Thieme; 2008; 88.
Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A 2003;100:5057–62. doi:10.1073/pnas.08309181000830918100.
Lassmann M, Hänscheid H, Gassen D, Biko J, Meineke V, Reiners C, et al. In vivo formation of gamma-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J Nucl Med 2010;51:1318–25. doi:10.2967/jnumed.109.071357.
Andrievski A, Wilkins RC. The response of gamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. Int J Radiat Biol 2009;85:369–76. doi:10.1080/09553000902781147.
Sgouros G, Knox SJ, Joiner MC, Morgan WF, Kassis AI. MIRD continuing education: bystander and low dose-rate effects: are these relevant to radionuclide therapy? J Nucl Med 2007;48:1683–91. doi:10.2967/jnumed.105.028183.
Sokolov MV, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication. Cell Cycle 2007;6:2210–2.
Chin BB, Green ED, Turkington TG, Hawk TC, Coleman RE. Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol 2009;11:118–22. doi:10.1007/s11307-008-0177-9.
Kuefner MA, Grudzenski S, Hamann J, Achenbach S, Lell M, Anders K, et al. Effect of CT scan protocols on x-ray-induced DNA double-strand breaks in blood lymphocytes of patients undergoing coronary CT angiography. Eur Radiol 2010;20:2917–24. doi:10.1007/s00330-010-1873-9.
Rothkamm K, Balroop S, Shekhdar J, Fernie P, Goh V. Leukocyte DNA damage after multi-detector row CT: a quantitative biomarker of low-level radiation exposure. Radiology 2007;242:244–51. doi:10.1148/radiol.2421060171.
Rothkamm K, Horn S. gamma-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita 2009;45:265–71.
Rübe CE, Grudzenski S, Kühne M, Dong X, Rief N, Löbrich M, et al. DNA double-strand break repair of blood lymphocytes and normal tissues analysed in a preclinical mouse model: implications for radiosensitivity testing. Clin Cancer Res 2008;14:6546–55. doi:10.1158/1078-0432.CCR-07-5147.
Golfier S, Jost G, Pietsch H, Lengsfeld P, Eckardt-Schupp F, Schmid E, et al. Dicentric chromosomes and gamma-H2AX foci formation in lymphocytes of human blood samples exposed to a CT scanner: a direct comparison of dose response relationships. Radiat Prot Dosimetry 2009;134:55–61. doi:10.1093/rpd/ncp061.
Acknowledgments
We thank Christina Engert, Tobias Löwe, Matthias Sommer, Bernhard Schmidt and Wolfram Nitsch for excellent technical assistance and Luitpold Distel for extraordinary collaboration in the lab. We also thank Ulrich Kahl, Ulrich Gärtner, Gerson Schütze, Kilian Bose, Rainer Linke and Roland Wondra for their support in patient examination and acquisition of blood samples.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
May, M.S., Brand, M., Wuest, W. et al. Induction and repair of DNA double-strand breaks in blood lymphocytes of patients undergoing 18F-FDG PET/CT examinations. Eur J Nucl Med Mol Imaging 39, 1712–1719 (2012). https://doi.org/10.1007/s00259-012-2201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2201-1