Abstract
Purpose
Invasive pulmonary aspergillosis is mainly caused by Aspergillus fumigatus, and is one of the major causes of morbidity and mortality in immunocompromised patients. The mortality associated with invasive pulmonary aspergillosis remains high, mainly due to the difficulties and limitations in diagnosis. We have shown that siderophores can be labelled with 68Ga and can be used for PET imaging of A. fumigatus infection in rats. Here we report on the further evaluation of the most promising 68Ga-siderophore candidates, triacetylfusarinine (TAFC) and ferrioxamine E (FOXE).
Methods
Siderophores were labelled with 68Ga using acetate buffer. Log P, protein binding and stability values were determined. Uptake by A. fumigatus was studied in vitro in cultures with high and low iron loads. In vivo biodistribution was determined in normal mice and an infection model was established using neutropenic rats inoculated with A. fumigatus. Static and dynamic μPET imaging was performed and correlated with CT images, and lung infection was evaluated ex vivo.
Results
68Ga-siderophores were labelled with high radiochemical purity and specific activity. 68Ga-TAFC and 68Ga-FOXE showed high uptake by A. fumigatus in iron-deficient cultures. In normal mice, 68Ga-TAFC and 68Ga-FOXE showed rapid renal excretion with high metabolic stability. In the rat infection model focal lung uptake was detected by μPET with both compounds and increased with severity of the infection, correlating with abnormal CT images.
Conclusion
68Ga-TAFC and 68Ga-FOXE displayed excellent in vitro stability and high uptake by A. fumigatus. Both compounds showed excellent pharmacokinetics, highly selective accumulation in infected lung tissue and good correlation with severity of disease in a rat infection model, which makes them promising agents for A. fumigatus infection imaging.
Similar content being viewed by others
Introduction
Invasive fungal diseases are among the leading causes of morbidity and mortality in haematopoietic stem cell and solid organ transplant recipients, as well as in patients with solid tumours and haematological malignancies [1–3]. In recent years, an increase (from 19 % to 25 %) in the incidence of infections caused by opportunistic mould pathogens including Aspergillus, Candida, zygomycete, Fusarium, Scedosporium and Acremonium species has been observed [1, 3, 4], with invasive aspergillosis (IA) being the predominant infection [5, 6]. The mortality rate associated with IA, which is mainly caused by Aspergillus fumigatus and primarily affects the lungs, remains unacceptably high (30–95 %) [2, 3, 7]. Singh et al. have reported that an estimated 9.3–16.9 % of all deaths in transplant recipients in the first year can be attributable to IA [8, 9].
Early and accurate diagnosis of IA is critical for a favourable outcome, but is difficult to achieve with currently available methods [10, 11]. Current methods for the diagnosis of IA include prognostic factors, clinical signs, radiology and laboratory tests (e.g. galactomannan antigen, PCR, microscopy and culture) [10, 11]. However, most of these techniques lack sufficient specificity and/or sensitivity for early detection of IA. Identification of patients at high risk, appropriate prophylaxis, diagnostic surveillance, and early diagnosis remain important for improved patient management [11], and underline the need for specific and sensitive imaging methods for IA.
Iron is an essential nutrient and is also a key factor in the virulence of pathogenic microorganisms [12, 13]. In response to low iron availability, iron-dependent microorganisms have evolved different strategies to obtain iron. These strategies include the biosynthesis of low molecular mass iron chelators, termed siderophores, with extremely high affinity for ferric ions, which are employed for iron delivery by almost all bacteria and fungi (including A. fumigatus) as well as in some plants [14]. The major siderophore produced by A. fumigatus for iron acquisition is triacetylfusarinine C (TAFC). The importance of TAFC for A. fumigatus during virulence is reflected by the transcriptional upregulation of its biosynthesis and uptake during infection as well as the attenuation of virulence by inactivation of TAFC biosynthesis in murine IA models [15, 16]. Aspergillus recovers iron from iron-siderophore complexes via specific uptake mechanisms involving highly efficient siderophore transporters [17]. Remarkably, numerous fungi including Aspergillus species possess specific uptake systems not only for native siderophores, but also for siderophores synthesized exclusively by other fungi [18].
68Ga is a positron emitter that has recently become the subject of great interest for molecular imaging applications using PET [19]. It is readily available from a 68Ge/68Ga generator and has a suitably short half-life of 68 min. In addition, Ga3+ has comparable complex chemistry to Fe3+, and binds with high affinity to siderophores [20].
In a proof of principle study, we recently showed that a 68Ga-labelled TAFC can detect A. fumigatus infection in a rat animal model using PET imaging [20]. In a subsequent study, we characterized the in vitro and in vivo behaviour of selected siderophores [21], showing that besides 68Ga-TAFC, 68Ga-ferrioxamine E (68Ga-FOXE) also shows high uptake by A. fumigatus in culture and remains stable in vivo. In this study we compared these most promising candidates including investigations in a rat A. fumigatus infection model and μPET imaging.
Materials and methods
Chemicals
All commercially available reagents were of analytical grade and used without further purification. Siderophores were obtained from Genaxxon Bioscience (Ulm, Germany). 68Ga was eluted from a 68Ge/68Ga generator (IGG; Eckert & Ziegler, Berlin, Germany).
Radiolabelling and in vitro studies
TAFC and FOXE were labelled with 68Ga using acetate buffer at room temperature for 15 min (TAFC) and at 80 °C for 20 min (FOXE). For all in vitro and in vivo studies, the pH of the final product was adjusted with 1.1 M sodium acetate to pH 6–7. The radiochemical purity, log P, protein binding and stability of 68Ga-siderophores in various media were determined, as described previously [20].
Preparation of A. fumigatus cultures for in vitro uptake studies
The Aspergillus strain used for in vitro studies was A. fumigatus wild-type ATCC46645 (American Type Culture Collection) cultured at 37 °C in Aspergillus minimal medium, containing 1 % glucose as the carbon source, 20 mM glutamine as the nitrogen source, salts and trace elements, as described previously [22]. Iron-sufficient media contained 30 mM FeSO4. For preparation of iron-deficient media, iron addition was omitted. Iron-deficient conditions were verified by detection of extracellular siderophore production, which is suppressed by iron.
In vitro uptake of 68Ga-siderophores by A. fumigatus
Uptake by A. fumigatus in iron-deficient and iron-sufficient cultures was studied. For the monitoring of uptake over time, 68Ga-siderophores (5 ng) were incubated in microbial media for 10, 20, 30, 45, 60 and 90 min at room temperature in 96-well plates (Millipore, Billerica, MA). For the monitoring of uptake blocking, excess of ferri-siderophore (Fe-TAFC or Fe-FOXE) and/or sodium azide was used. 68Ga-siderophores were incubated in iron-deficient and iron-sufficient media for 45 min at room temperature in 96-well plates. The incubation was interrupted in both cases by filtration of the medium and rapid rinsing with ice-cold TRIS buffer. The filters were collected and counted in a γ-counter.
Preparation of A. fumigatus inoculum for rat infection model
The A. fumigatus (A29) isolate was grown on Sabouraud dextrose agar (BD) for 5 days at 37 °C, and the conidia were harvested in 2 ml of sterile NaCl by gently rubbing with a pipette tip. The conidia suspension was transferred into a sterile 50 ml plastic tube. After homogenization (vortex) and filtration (40 μm nylon cell strainer; BD), the suspension was counted in a Neubauer chamber and adjusted to the volitional concentration in the range 1 × 105 to 1 × 109 conidia per millilitre.
Animal experiments
All animal experiments were conducted in accordance with regulations and guidelines of the Austrian and Dutch animal Protection laws and with the approval of the Austrian Ministry of Science (66011/42-II/10b/2009), and the institutional Animal Welfare Committee of the Radboud University Medical Centre Nijmegen (revised Dutch Act on Animal Experimentation, 1997). Animal studies were performed using Balb/c mice and Lewis rats (both Charles River Laboratories, Wilmington, MA).
Biodistribution in normal mice
Normal noninfected Balb/c mice (female, 6 weeks old) were injected with 68Ga-siderophore (2 MBq and 0.1–0.2 μg of siderophore per mouse) into the tail vein. Animals were killed by cervical dislocation 30 min and 90 min after injection. The organs and tissues (blood, spleen, pancreas, stomach, intestines, kidneys, liver, heart, lungs, muscle and femur) were removed and radioactivity was counted in a γ-counter. The results are expressed as percentage of injected dose per gram of tissue.
Metabolic stability
Urine, blood, liver and kidneys of normal Balb/c mice injected with 68Ga-siderophores and treated as described previously were collected 30 min after injection. The urine sample was directly injected onto the RP-HPLC column. Blood samples were precipitated with acetonitrile and centrifuged for 2 min, and the supernatant was injected onto the RP-HPLC column. Liver and kidneys were washed in the ice-cold TRIS buffer and liquidized using a mixer in a falcon tube containing 1 ml of TRIS buffer. The liver and kidney homogenates obtained were mixed with acetonitrile and centrifuged for 2 min, and the supernatant was injected onto the RP-HPLC column. In all cases 1 min fractions of the column eluate were collected and measured in a γ-counter. Samples were not collected at 90 min after injection because the measured activity was already low in the samples obtained 30 min after injection. All metabolic studies were performed using a previously described HPLC method [20].
Rat infection models
Standard
Female or male Lewis rats (2–3 months old), weighing 200–250 g, were treated as described previously [20]. Briefly, the rats received repeated intraperitoneal injections of cyclophosphamide to induce neutropenia before A. fumigatus administration. To prevent bacterial superinfection, the animals were given antibiotics throughout the experiment. Fungal infection was established by intratracheal administration of 100–300 μl of A. fumigatus inoculum in various concentrations (1 × 105 to 1 × 109 conidia per millilitre). Animals were injected intravenously 1–3 days (depending on the severity of infection) after A. fumigatus administration with 68Ga-labelled siderophore (10–20 MBq and 1–2 μg of siderophore per rat). The rats were imaged or killed by overdosing with thiopental (Sandoz, Kundl, Austria) 2 h after injection. Various organs and tissues (blood, spleen, kidneys, liver and lungs) were removed and radioactivity was measured in a γ-counter. The excised organs were investigated for the presence of fungi, as described below.
Iron preload
Rats were treated as in the ‘standard’ infection model above, except that iron solution (FerMed; Medice, Iserlohn, Germany) 10 mg/kg was injected intraperitoneally three times (1 week, 4 days and 1 day) before A. fumigatus administration.
68Ga-siderophore imaging in the rat infection model
PET images were acquired with an Inveon animal PET/CT scanner (Siemens Preclinical Solutions, Knoxville, TN) with an intrinsic spatial resolution of 1.5 mm [23]. The animals were placed in a prone position. Static PET images were acquired over 30 min starting 30 min after intravenous injection of 68Ga-siderophore. Dynamic PET imaging was started upon injection and continued up to 60 min after injection. In addition, combined PET/CT scans were performed for anatomical reference. PET emission scans were acquired for 30 min, preceded by CT scans (spatial resolution 113 μm, 80 kV, 500 μA, exposure time 300 ms). After imaging, animals were killed by CO2/O2. Scans were reconstructed using Inveon Acquisition Workplace software (version 1.5; Siemens Preclinical Solutions, Knoxville, TN) using a 3-D ordered subset expectation maximization/maximum a posteriori (OSEM3D/MAP) algorithm with the following parameters: matrix 256 × 256 × 159, pixel size 0.43 × 0.43 × 0.8 mm3 and a MAP prior β-value of 1.5.
In vitro cultures of excised organs
The excised organs were homogenized in a petri dish using a sterile surgical blade and transferred to Sabouraud dextrose agar (BD) plates. The plates were incubated at 37 °C and examined daily for 7 days. Colony-forming counts were recorded from all plates that showed growth. Severe infection was defined as the presence of severe fungal growth 1 day after incubation, mild infection was defined as the presence of minor growth up to 3 days after incubation, and no infection was defined as lack of growth within 1 week of incubation.
Statistical analysis
Student’s t-test (level of significance, P < 0.05) was used to determine the significance of differences in the ex vivo and in vivo data. Analysis was performed using Microsoft Office Excel 2007.
Results
Radiolabelling and in vitro studies
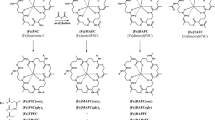
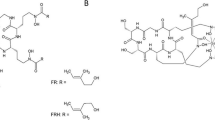
68Ga-TAFC and 68Ga-FOXE (Fig. 1) were both labelled with high radiochemical purity (≥95 %). High specific activity labelling was achieved up to 9.2 × 104 GBq/mmol for 68Ga-TAFC and 3.4 × 103 GBq/mmol for 68 Ga-FOXE. Both compounds showed hydrophilic properties (log P TAFC = −2.59, log P FOXE = −1.65) with low values of protein binding even 120 min after incubation in human serum (1.21 % for 68Ga-TAFC, 0.53 % for 68Ga-FOXE). The stability of the studied compounds was tested in various media – human serum, 0.1 M FeCl3 and 6 mM DTPA. 68Ga-FOXE showed excellent stability (≥90 %) in all of the tested media. 68Ga-TAFC displayed comparable results, with the lowest stability (≥80 %) in 6 mM DTPA 120 min after incubation. A summary of the in vitro characteristics of both 68Ga-siderophores is given in Online Resource 1.
In vitro uptake of 68Ga-siderophores by A. fumigatus
Uptake of 68Ga-TAFC and 68Ga-FOXE was highly dependent on the mycelial iron load. Both compounds showed rapid uptake by A. fumigatus in iron-deficient cultures, which could be blocked with excess of ferri-siderophore and/or sodium azide and was significantly lower (P < 0.05) in iron-sufficient media. Figure 2 shows 68Ga-TAFC and 68Ga-FOXE specific uptake over time (Fig. 2a) and specific uptake 45 min after incubation (Fig. 2b), which could be blocked with excess of ferri-siderophore and/or sodium azide in the A. fumigatus cultures.
Biodistribution in normal Balb/c mice
In normal noninfected mice (Online Resource 2), 68Ga-TAFC showed rapid renal elimination and low blood levels as early as 30 min after injection (1.6 ± 0.37 %ID/g at 30 min). In a similar way, 68Ga-FOXE displayed fast renal elimination and relatively low blood levels 30 min after injection (2.4 ± 0.86 %ID/g). The main difference in biodistribution of the studied 68Ga-siderophores was in the significantly higher (P < 0.05) intestinal accumulation (see also liver values in Online Resource 2) of 68Ga-FOXE as compared to that of 68Ga-TAFC (4.6 ± 1.42 %ID/g at 30 min and 2.4 ± 0.85 %ID/g at 90 min for 68Ga-FOXE vs. 1.7 ± 1.04 %ID/g at 30 min and 1.0 ± 0.26 %ID/g at 90 min for 68Ga-TAFC).
Metabolic stability
Both compounds showed high metabolic stability in urine, blood and kidney homogenate. In all cases almost only intact 68Ga-TAFC and 68Ga-FOXE were detected (the lowest values of administered 68Ga-TAFC and 68Ga-FOXE were 96.7 % and 95.9 % in kidney homogenate and urine, respectively). The major difference in the stability between the two 68Ga-siderophores was observed in liver homogenate. 68Ga-FOXE showed significantly higher (P < 0.05) metabolism than 68Ga-TAFC (68Ga-FOXE 71.3 % intact vs. 68Ga-TAFC 98.1 % intact on HPLC analysis). A summary of metabolic stabilities of the studied 68Ga-siderophores is given in Online Resource 3.
Biodistribution of 68Ga-siderophores in rats – A. fumigatus infection model
In summary, 20 rats were treated with 68Ga-TAFC and 19 rats with 68Ga-FOXE. For 68Ga-TAFC, five rats developed severe and five rats mild lung infection, and ten rats showed no signs of infection. In the case of 68Ga-FOXE, six rats developed severe lung infection and six rats mild lung infection, and seven rats showed no sign of infection. In the severely infected animals, high levels of 68Ga-siderophores accumulated in the infected lungs, whereas in the noninfected animals, 68Ga-siderophores were rapidly excreted via the kidneys with low levels of accumulation in other organs. A significant difference (P < 0.05) between mildly infected and noninfected rats was observed. Figure 3 shows lung uptake values in the different groups of rats 2 h after administration of 68Ga-siderophores. The highest uptake was observed in severely infected rats injected with 68Ga-FOXE (3.45 ± 1.00 %ID/g, n = 5), followed by 0.95 ± 0.37 %ID/g (n = 4) in severely infected rats injected with 68Ga-TAFC. In the group of mildly infected animals, 68Ga-FOXE again showed slightly higher uptake 0.48 ± 0.54 %ID/g (n = 4) in comparison with 68Ga-TAFC (0.29 ± 0.12 %ID/g; n = 3). The control group showing no infection displayed similar results for both 68Ga-siderophores (0.04 ± 0.02 %ID/g for 68Ga-FOXE, n = 5; 0.04 ± 0.01 %ID/g for 68Ga-TAFC, n = 9). Rats pretreated with iron showed a comparable dependence of uptake on the severity of infection, with absolute values being somewhat lower than in the nonpretreated group, in particular for 68Ga-FOXE; however, none of the differences was statistically significant (P < 0.05). Online Resource 4 shows a comparison of lung uptake and target/non target ratios of 68Ga-siderophores in the different rat infection models.
68Ga-siderophore imaging in rats
MicroPET imaging in the rat infection model showed rapid focal accumulation of 68Ga-siderophores in the lungs, which increased with the severity of infection (Fig. 4). No uptake in the lung region was detected in noninfected animals in which the only visible organs were the kidneys and bladder (Fig. 4). Dynamic imaging (see Fig. 5 for 68Ga-FOXE) in severely infected rats revealed uptake in the infected lung area as early as 10–20 min after injection with improved contrast over time without detectable washout over the whole imaging period (60 min), whereas the activity rapidly accumulated in kidneys and decreased over time. Figure 6 shows a correlation between PET and CT scans for both compounds under study. In CT scans of severely infected rats the changes in infected tissue were visible as grey areas in the lung region that fully corresponded with radioactivity lung accumulation in PET scans. Fusing images of both modalities revealed matching uptake, clearly visible in the fused images. Target/non-target ratios as well as SUV values in infected animals were calculated from all imaging studies performed (n = 13) and were 5.81 ± 6.05 and 0.78 ± 0.75 for 68Ga-TAFC and 6.64 ± 2.91 and 1.00 ± 0.81 for 68Ga-FOXE, respectively (see Online Resource 5), showing no significant difference (P < 0.05) between the two compounds in terms of quantitative uptake behaviour.
Static PET scans (30 min after injection) in severely infected rats (left), mildly infected rats (centre) and noninfected rats (right) show different levels of accumulation of 68Ga-TAFC (a) and 68Ga-FOXE (b) in infected lungs (arrows) depending on the severity of infection. No infection in non-infected animals is seen
Discussion
The need for novel approaches to the imaging of IA is reflected by the number of radiopharmaceuticals that have been described and proposed for this application [24, 25]. Radiopharmaceuticals for which clinical applications have been proposed include 67Ga-citrate [26], but it has known limitations in terms of pharmacokinetics, sensitivity and specificity, as it is a general marker for imaging malignancies and inflammatory processes. Even though 18F-FDG has also recently been proposed as an imaging agent for IA [27], it has comparable limitations, in particular related to the low specificity for imaging glucose metabolism. Various attempts have been made to develop more specific radiopharmaceuticals for this application, including 99mTc-labelled polyethyleneglycol liposomes [28], 99mTc-interleukin-8 [29], 99mTc-fluconazole [30] and 99mTc-antimicrobial peptides (e.g. ubiquicidin) [30, 31]. None of these agents has proven to show specific uptake mechanisms in Aspergillus species and none has entered clinical trials. Recently a hypha-binding peptide (c(CGGRLGPFC)-NH2) labelled with 111In has been described [32] potentially having higher specificity, but further evaluation towards clinical application has not been reported.
The use of a 68Ga-labelled siderophore that is actively taken up via specific iron transporters by the microorganism acquiring iron during the course of infection holds the potential of a unique and specific way to image IA. In a proof of principle study [20], we have shown that siderophores can be labelled with 68Ga with high affinity and stability in biological systems. In vitro energy-dependent uptake of 68Ga-siderophores in A. fumigatus was observed and preliminary in vivo studies were performed, proving the potential of 68Ga-labelled siderophores for infection imaging. After the promising study with 68Ga-TAFC in the rat infection model, we focused on the selection, characterization and optimization of the most promising candidates for diagnostic applications as a basis for clinical implementation of PET (PET/CT) in imaging of fungal infections [21].
In this study we compared two 68Ga-labelled siderophores as the most promising candidates from previous studies [20, 21] and evaluated their potential as radiopharmaceuticals for IA imaging. Both 68Ga-FOXE and 68Ga-TAFC showed hydrophilic properties, low protein binding and high in vitro stability. In vitro studies showed rapid and high uptake by A. fumigatus in iron-deficient media, which could be blocked with excess of ferri-siderophore or sodium azide. Both compounds showed excellent pharmacokinetic properties with high metabolic stability. Nevertheless, 68Ga-FOXE showed significantly lower metabolic stability in the liver than 68Ga-TAFC, which could explain the higher accumulation of radioactivity in the liver and intestinal tissue observed in biodistribution studies of Balb/c mice. This was confirmed in μPET imaging of rats. Both 68Ga-siderophores showed highly selective accumulation in infected lung which was shown to be correlated with severity of disease in the rat infection model using μPET or μPET/CT. Even though 68Ga-FOXE had higher uptake values in biodistribution studies indicating a potentially higher sensitivity, PET/CT imaging did not show significant differences either in uptake or in target/non-target ratios.
Today CT is the standard imaging technique for the detection of pulmonary infections [33, 34], and previous studies have shown that the halo sign is indicative of pulmonary aspergillosis in neutropenic patients [35]. However, CT scans may not allow differentiation between Aspergillus and other pathogenic fungi [36] and CT has limited specificity and predictive value, especially in non-neutropenic stem-cell transplant recipients [36, 37]. The combination with PET could provide additional functional information on the lesion detected, thereby increasing sensitivity and specificity of patient imaging within one diagnostic procedure. The effectiveness of combined PET and CT imaging is illustrated in Fig. 6. The PET images of both 68Ga-siderophores in rats infected with A. fumigatus show abnormal uptake of radiotracer in the thoracic area. The fused PET/CT image permits precise localization of the lung tissue affected by A. fumigatus infection. We found matching uptake in pathological areas on CT images with the accumulation of our investigated 68Ga-siderophores in the infected lung areas. The combination of PET and 68Ga-labelled siderophores (TAFC or FOXE) and CT therefore holds potential for early detection of invasive fungal infections.
In the clinical setting, one of the risk factors for IA in immunocompromised patients is a high iron load [38], which is frequently occurs due to blood transfusions or iron supplementation during the course of their underlying disease. As 68Ga-siderophores mimic iron-transporting mechanisms in microorganisms, we wanted to see whether an iron preload would have an effect on the uptake of 68Ga-labelled siderophores in the IA rat model. In the small series of animals tested, no significant decrease in uptake of either 68Ga-TAFC or 68Ga-FOXE in fungal infection could be observed indicating that iron supply does not influence uptake of the tracers. Another important factor in judging the suitability of this imaging approach is the selectivity of 68Ga-siderophores for fungal infections. We are currently investigating this issue in an ongoing study to determine the uptake of these 68Ga-siderophores by a variety of microorganisms, which will help in choosing the optimal candidate for noninvasive detection of fungal infections by PET in a clinical setting.
Conclusion
Our study showed that both 68Ga-labelled TAFC and FOXE are very promising agents for detection of IA with high sensitivity. The high metabolic stability, favourable pharmacokinetics with rapid renal excretion and high specific uptake in A. fumigatus cultures were confirmed in imaging studies in a rat IA model that showed high focal uptake in infected lung tissue corresponding to pathological findings seen on CT. 68Ga-TAFC showed advantages in terms of radiolabelling and a somewhat higher metabolic stability, and 68Ga-FOXE showed a trend towards higher uptake in infected tissue. Only currently ongoing selectivity studies will enable selection of the optimal candidate for potential clinical applications.
References
Pagano L, Akova M, Dimopoulos G, Herbrecht R, Drgona L, Blijlevens N. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J Antimicrob Chemother. 2011;66:i5–14.
Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;50:1559–67.
Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica. 2006;91:986–9.
Steinbach WJ, Reedy JL, Cramer RA, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5:418–30.
Kontoyiannis DP, Bodey GP. Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis. 2002;21:161–72.
Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803.
Hope WW, Walsh TJ, Denning DW. The invasive and saprophytic syndromes due to Aspergillus spp. Med Mycol. 2005;43:207–38.
Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69.
Singh N, Husain S; AST Infectious Diseases Community of Practice. Invasive aspergillosis in solid organ transplant recipients. Am J Transplant. 2009;9:180–91.
Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22.
Reichenberger F, Habicht JM, Gratwohl A, Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19:743–55.
Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32:70–8.
Sritharan M. Iron and bacterial virulence. Indian J Med Microbiol. 2006;24:163–4.
Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27:637–57.
McDonagh A, Fedorova ND, Crabtree J, Yu Y, Kim S, Chen D, et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008;4:e1000154.
Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, et al. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007;3:1195–207.
Schrettl M, Kim HS, Eisendle M, Kragl C, Nierman WC, Heinekamp T, et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol Microbiol. 2008;70:27–43.
Haas H. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl Microbiol Biotechnol. 2003;62:316–30.
Fani M, André JP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging. 2008;3:53–63.
Petrik M, Haas H, Dobrozemsky G, Lass-Flörl C, Helbok A, Blatzer M, et al. 68Ga-siderophores for PET imaging of invasive pulmonary aspergillosis: proof of principle. J Nucl Med. 2010;51:639–45.
Petrik M, Haas H, Schrettl M, Helbok A, Blatzer M, Decristoforo C. In vitro and in vivo evaluation of selected (68)Ga-siderophores for infection imaging. Nucl Med Biol. 2012;39:361–9
Pontecorvo G, Roper JA, Hemmons LM, MacDonald KD, Bufton AW. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238.
Visser EP, Disselhorst JA, Brom M, Laverman P, Gotthardt M, Oyen WJ, et al. Spatial resolution and sensitivity of the Inveon small-animal PET scanner. J Nucl Med. 2009;50:139–47.
Lupetti A, de Boer MGJ, Erba P, Campa M, Nibbering PH. Radiotracers for fungal infection imaging. Med Mycol. 2011;49:62–9.
Boerman OC, Dams ET, Oyen WJ, Corstens FH, Storm G. Radiopharmaceuticals for scintigraphic imaging of infection and inflammation. Inflamm Res. 2001;50:55–64.
Del Val Gomez M, Gallardo F, Babe J, Cobo J. Ga-67 scan in invasive aspergillosis: a case report. Clin Nucl Med. 2000;25:1035–6.
Hot A, Maunoury C, Poiree S, Lanternier F, Viard JP, Loulergue P, et al. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin Microbiol Infect. 2011;17:409–17.
Boerman OC, Laverman P, Oyen WJ, Corstens FH, Storm G. Radiolabeled liposomes for scintigraphic imaging. Prog Lipid Res. 2000;39:461–75.
Rennen HJ, Bleeker-Rovers CP, van Eerd JE, Frielink C, Oyen WJ, Corstens FH, et al. 99mTc-labeled interleukin-8 for scintigraphic detection of pulmonary infections. Chest. 2004;126:1954–61.
Lupetti A, Welling MM, Mazzi U, Nibbering PH, Pauwels EK. Technetium-99m labelled fluconazole and antimicrobial peptides for imaging of Candida albicans and Aspergillus fumigatus infections. Eur J Nucl Med Mol Imaging. 2002;29:674–9.
Welling MM, Paulusma-Annema A, Balter HS, Pauwels EKJ, Nibbering PH. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med. 2000;27:292–301.
Yang Z, Kontoyiannis DP, Wen X, Xiong C, Zhang R, Albert ND, et al. Gamma scintigraphy imaging of murine invasive pulmonary aspergillosis with a 111In-labeled cyclic peptide. Nucl Med Biol. 2009;36:259–66.
Marom EM, Kontoyiannis PD. Imaging studies for diagnosing invasive fungal pneumonia in immunocompromised patients. Curr Opin Infect Dis. 2011;24:309–14.
Munoz P, Guinea J, Bouza E. Update on invasive aspergillosis: clinical and diagnostic aspects. Clin Microbiol Infect. 2006;12:24–39.
Caillot D, Casasnovas O, Bernard A, Couaillier JF, Durand C, Cuisenier B, et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–47.
Maertens J, van Eldere J, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002;186:1297–306.
Maschmeyer G. Pneumonia in febrile neutropenic patients: radiologic diagnosis. Curr Opin Oncol. 2001;13:229–35.
Deeg HJ, Spaulding E, Shulman HM. Iron overload, hematopoietic cell transplantation, and graft-versus-host disease. Leuk Lymphoma. 2009;50:1566–72.
Acknowledgments
We would like to thank the staff of the Central Laboratory Animal Facilities in Innsbruck and Nijmegen and we especially thank Bianca Lemmers and Kitty Lemmens for excellent help in the animal experiments. We acknowledge the financial support of the Austrian Science Foundation (FWF; grant L676-B18 B18 to C.D. and P-21643-B11 to H.H.).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below are the links to the electronic supplementary material.
Online Resource 1
In vitro characteristics of 68Ga-TAFC and 68Ga-FOXE (DOCX 15.8 kb)
Online Resource 2
Biodistribution of 68Ga-TAFC and 68Ga-FOXE in normal (noninfected) mice 30 and 90 min after injection (DOCX 57 kb)
Online Resource 3
Metabolic stability of 68Ga-TAFC and 68Ga-FOXE in normal Balb/c mice 30 min after injection. (DOCX 14 kb)
Online Resource 4
Comparison of lung uptake and ratios in organs of interest of 68Ga-siderophores in different groups of rat infection model (DOCX 17.0 kb)
Online Resource 5
Comparison of target/non-target (lung) ratios and SUVs of the studied 68Ga-siderophores in infected rats (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Petrik, M., Franssen, G.M., Haas, H. et al. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur J Nucl Med Mol Imaging 39, 1175–1183 (2012). https://doi.org/10.1007/s00259-012-2110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2110-3