Abstract
Purpose
To identify the neurobiological traits of amyotrophic lateral sclerosis (ALS) and to elucidate functional differences between ALS of spinal and bulbar onset. We hypothesized that glucose metabolism distribution might vary between groups.
Methods
The study groups comprised 32 patients with ALS of either bulbar (n = 13) or spinal (n = 19) onset and 22 subjects as controls. They were investigated by [18F]fluorodeoxyglucose (FDG) positron emission tomography (FDG PET), comparing the patient groups with each other and with the controls by statistical parametric mapping.
Results
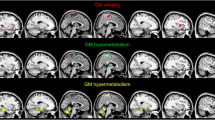
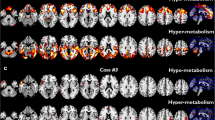
Highly significant relative increases in glucose metabolism distribution were found in the group comprising all 32 ALS patients as compared with the controls in the bilateral amygdalae, midbrain, pons and cerebellum. Relative hypermetabolism was also found in patients with spinal onset as compared with the controls in the right midbrain. In patients with bulbar onset compared with the controls and with patients with spinal onset, large relatively hypometabolic areas were found in the bilateral frontal cortex, right insula, anterior cingulate, precuneus and inferior parietal lobe. Patients with spinal onset had significantly higher scores in a neuropsychological test assessing verbal fluency compared with patients with bulbar onset.
Conclusion
This large FDG PET investigation provided unprecedented evidence of relatively increased metabolism in the amygdalae, midbrain and pons in ALS patients as compared with control subjects, possibly due to local activation of astrocytes and microglia. Highly significant relative decreases in metabolism were found in large frontal and parietal regions in the bulbar onset patients as compared with the spinal onset patients and the controls, suggesting a differential metabolic and neuropsychological state between the two conditions.



Similar content being viewed by others
References
Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
Chiò A, Mora G, Calvo A, Mazzini L, Bottacchi E, Mutani R, et al. Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology. 2009;72:725–31.
Leigh PN, Kew JJM, Goldstein LH, Brooks DJ. The cerebral lesions in amyotrophic lateral sclerosis: new insights from pathology and functional brain imaging. In: Rose C, editor. Amyotrophic lateral sclerosis from Charcot to the present and into the future. London: Smith-Gordon; 1994. p. 191–209.
Talbot PR, Goulding PJ, Lloyd JJ, Snowden JS, Neary D, Testa HJ. Inter-relation between ‘classic’ motor neuron disease and frontotemporal dementia: neuropsychological and single photon emission computed tomography study. J Neurol Neurosurg Psychiatry. 1995;58:541–7.
Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, et al. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65:636–41.
Gallassi R, Montagna P, Morreale A, Lorusso S, Tinuper P, Daidone R, et al. Neuropsychological, electroencephalogram and brain computed tomography findings in motor neuron disease. Eur Neurol. 1989;29:115–20.
Ludolph AC, Langen KJ, Regard M, Herzog H, Kemper B, Kuwert T, et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand. 1992;85:81–9.
Iwasaki Y, Kinoshita M, Oceda K, Takamiya K, Shiojima T. Cognitive impairment in amyotrophic lateral sclerosis and its relation to motor disabilities. Acta Neurol Scand. 1990;81:141–3.
Kew JJM, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, et al. The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain. 1993;116:1399–423.
Abrahams S, Leigh PN, Kew JJ, Goldstein LH, Lloyd CM, Brooks DJ. A positron emission tomography study of frontal lobe function (verbal fluency) in amyotrophic lateral sclerosis. J Neurol Sci. 1995;129(Suppl):44–6.
Abe K, Fujimura H, Toyooka K, Sakoda S, Yorifuji S, Yanagihara T. Cognitive function in amyotrophic lateral sclerosis. J Neurol Sci. 1997;148:95–100.
Abrahams S, Goldstein LH, Suckling J, Ng V, Simmons A, Chitnis X, et al. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol. 2005;252:321–31.
Andreadou E, Sgouropoulos P, Varelas P, Gouliamos A, Papageorgiou C. Subcortical frontal lesions on MRI in patients with motor neurone disease. Neuroradiology. 1998;40:298–302.
Kato S, Hayashi H, Yagishita A. Involvement of the frontotemporal lobe and limbic system in amyotrophic lateral sclerosis: as assessed by serial computed tomography and magnetic resonance imaging. J Neurol Sci. 1993;116:52–8.
Peavy GM, Herzog AG, Rubin NP, Mesulam M-M. Neuropsychological aspects of dementia of motor neuron disease: a report of two cases. Neurology. 1992;42:1004–8.
Hudson AJ. Amyotrophic lateral sclerosis and its association with dementia, parkinsonism and other neurological disorders: a review. Brain. 1981;104:217–47.
Dalakas MC, Hatazawa J, Brooks RA, Di Chiro G. Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann Neurol. 1987;22:580–6.
Hatazawa J, Brooks RA, Dalakas MC, Mansi L, Di Ghiro G. Cortical moto-sensory hypometabolism in amyotrophic lateral sclerosis: a PET study. J Comput Assist Tomogr. 1988;12:630–6.
Le Forestier N, Maisonobe T, Spelle L, Lesort A, Salachas F, Lacomblez L, et al. Primary lateral sclerosis: further clarification. J Neurol Sci. 2001;185:95–100.
Turner MR, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, Brooks DJ, et al. Cortical involvement in four cases of primary lateral sclerosis using [(11)C]-flumazenil PET. J Neurol. 2007;254:1033–6.
Claassen DO, Josephs KA, Peller PJ. The stripe of primary lateral sclerosis: focal primary motor cortex hypometabolism seen on fluorodeoxyglucose F18 positron emission tomography. Arch Neurol. 2010;67:122–5.
Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioral syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2009;10:131–46.
Bennett CM, Wolford GL, Miller MB. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 2009;4:417–22.
Yatham LN, Liddle PF, Lam RW, Zis AP, Stoessl AJ, Sossi V, et al. Effect of electroconvulsive therapy on brain 5-HT2 receptors in major depression. Br J Psychiatry. 2010;196:474–9.
Lloyd CM, Richardson MP, Brooks DJ, Al-Chalabi A, Leigh PN. Extramotor involvement in ALS: PET studies with the GABA(A) ligand [(11)C]flumazenil. Brain. 2000;123:2289–96.
Turner BJ, Lopes EC, Cheema SS. The serotonin precursor 5-hydroxytryptophan delays neuromuscular disease in murine familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:171–6.
Aquilonius SM, Jossan SS, Ekblom JG, Askmark H, Gillberg PG. Increased binding of 3H-L-deprenyl in spinal cords from patients with amyotrophic lateral sclerosis as demonstrated by autoradiography. J Neural Transm Gen Sect. 1992;89:111–22.
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–3.
Yu I, Inaji M, Maeda J, Okauchi T, Nariai T, Ohno T, et al. Glial cell-mediated deterioration and repair of the nervous system after traumatic brain injury in a rat model as assessed by positron emission tomography. J Neurotrauma. 2010;27:1463–75.
Marik J, Ogasawara A, Martin-McNulty B, Ross J, Flores JE, Gill HS, et al. PET of glial metabolism using 2-18F-fluoroacetate. J Nucl Med. 2009;50:982–90.
Yokokura M, Mori N, Yagi S, Yoshikawa E, Kikuchi M, Yoshihara Y, et al. In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2011;38:343–51.
Fukumoto D, Hosoya T, Nishiyama S, Harada N, Iwata H, Yamamoto S, et al. Multiparametric assessment of acute and subacute ischemic neuronal damage: a small animal positron emission tomography study with rat photochemically induced thrombosis model. Synapse. 2011;65:207–14.
Barros LF, Porras OH, Bittner CX. Why glucose transport in the brain matters for PET. Trends Neurosci. 2005;28:117–9.
Magistretti PJ. Cellular bases of functional brain imaging: insights from neuron-glia metabolic coupling. Brain Res. 2000;886:108–12.
Nehlig A, Coles J. Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia. 2007;55:1238–50.
Abe K, Fujimura H, Toyooka K, Hazama T, Hirono N, Yorifuji S, et al. Single-photon emission computed tomographic investigation of patients with motor neuron disease. Neurology. 1993;43:1569–73.
Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia. 2000;38:734–47.
Abrahams S, Goldstein LH, Kew JJM, Brooks DJ, Lloyd CM, Frith CD, et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain. 1996;119:2105–20.
Abrahams S, Goldstein LH, Al-Chalabi A, Pickering A, Morris RG, Passingham RE, et al. Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1997;62:464–72.
Pagani M, Dessi B, Morbelli S, Brugnolo A, Salmaso D, Piccini A, et al. MCI patients declining and not-declining at mid-term follow-up: FDG-PET findings. Curr Alzheimer Res. 2010;7:287–94.
Nobili F, Mazzei D, Dessi B, Morbelli S, Brugnolo A, Barbieri P, et al. Unawareness of memory deficit in amnestic MCI: FDG-PET findings. J Alzheimers Dis. 2010;22:993–1003.
Del Sole A, Clerici F, Chiti A, Lecchi M, Mariani C, Maggiore L, et al. Individual cerebral metabolic deficits in Alzheimer’s disease and amnestic mild cognitive impairment: an FDG PET study. Eur J Nucl Med Mol Imaging. 2008;35:1357–66.
Borghammer P, Cumming P, Aanerud J, Gjedde A. Artefactual subcortical hyperperfusion in PET studies normalized to global mean: lessons from Parkinson’s disease. Neuroimage. 2009;45:249–57.
Acknowledgments
The authors wish to thank the patients who kindly agreed to participate in this study, Ms Deborah Bertoluzzo and the personnel of IRMET for their valuable help in patient management, and Mrs Emanuela Enrico for English editing. This work was in part supported by Compagnia di San Paolo, Programma Neuroscienze 2008–2009 (to Consuelo Valentini e Andrea Calvo), and by the European Community’s Health Seventh Framework Programme (FP7/2007-2013) to Adriano Chiò (grant 259867).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00259-011-2029-0.
Rights and permissions
About this article
Cite this article
Cistaro, A., Valentini, M.C., Chiò, A. et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging 39, 251–259 (2012). https://doi.org/10.1007/s00259-011-1979-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1979-6