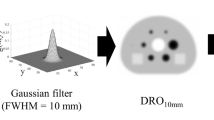
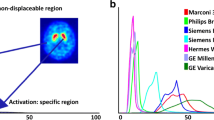
Abstract
Purpose
Multi-centre trials are an important part of proving the efficacy of procedures, drugs and interventions. Imaging components in such trials are becoming increasingly common; however, without sufficient control measures the usefulness of these data can be compromised. This paper describes a framework for performing high-quality multi-centre trials with single photon emission computed tomography (SPECT), using a pan-European initiative to acquire a normal control dopamine transporter brain scan database as an example.
Methods
A framework to produce high-quality and consistent SPECT imaging data was based on three key areas: quality assurance, the imaging protocol and system characterisation. Quality assurance was important to ensure that the quality of the equipment and local techniques was good and consistently high; system characterisation helped understand and where possible match the performance of the systems involved, whereas the imaging protocol was designed to allow a degree of flexibility to best match the characteristics of each imaging device.
Results
A total of 24 cameras on 15 sites from 8 different manufacturers were evaluated for inclusion in our multi-centre initiative. All results matched the required level of specification and each had their performance characterised. Differences in performance were found between different system types and cameras of the same type. Imaging protocols for each site were modified to match their individual characteristics to produce comparable high-quality SPECT images.
Conclusion
A framework has been designed to produce high-quality data for multi-centre SPECT studies. This framework has been successfully applied to a pan-European initiative to acquire a healthy control dopamine transporter image database.







Similar content being viewed by others
References
Geworski L, Knoop BO, de Wit M, Ivancević V, Bares R, Munz DL. Multicenter comparison of calibration and cross calibration of PET scanners. J Nucl Med 2002;43(5):635–9.
McEwan AS, Graham MM, Conti PS. Site qualification in multicenter clinical trials. J Nucl Med 2009;50(8):30N.
Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging 2007;34(3):392–404.
Scheuermann JS, Saffer JR, Karp JS, Levering AM, Siegel BA. Qualification of PET scanners for use in multicenter cancer clinical trials: the American College of Radiology Imaging Network experience. J Nucl Med 2009;50(7):1187–93.
Boellaard R, Oyen WJG, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging 2008;35(12):2320–33.
Graham MM. The Clinical Trials Network of the Society of Nuclear Medicine. Semin Nucl Med 2010;40(5):327–31.
McEwan AS, Graham MM, Conti PS. SNM molecular imaging summit introduces clinical trials network. J Nucl Med 2009;50(4):18N.
Kadrmas DJ, Casey ME, Conti M, Jakoby BW, Lois C, Townsend DW. Impact of time-of-flight on PET tumor detection. J Nucl Med 2009;50(8):1315–23.
Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 1997;62(2):133–40.
McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007;6(4):305–13.
Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord 2000;15(3):503–10.
Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord 2007;22(15):2210–5.
NEMA. NEMA NU 1: Performance measurements of gamma cameras [Internet]. 2007. Available via http://www.nema.org/stds/nu1.cfm.
IAEA. Chapter 8: SPECT systems. In: Quality control of nuclear medicine instrumentation. Vienna: IAEA; 1991. p. 253–300.
Darcourt J, Booij J, Tatsch K, Varrone A, Vander Borght T, Kapucu OL, et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging 2010;37(2):443–50.
Tossici-Bolt L, Dickson JC, Sera T, de Nijs R, Bagnara M, Jonsson C, et al. Calibration of gamma camera systems for a multicentre European (123)I-FP-CIT SPECT normal database. Eur J Nucl Med Mol Imaging 2011;38(8):1529–40.
Koch W, Radau PE, Hamann C, Tatsch K. Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med 2005;46(7):1109–18.
Christian PE. Ensuring standardization and harmonization in multicenter clinical trials. J Nucl Med 2009;50(5):20N.
Koch W, Radau PE, Münzing W, Tatsch K. Cross-camera comparison of SPECT measurements of a 3-D anthropomorphic basal ganglia phantom. Eur J Nucl Med Mol Imaging 2006;33(4):495–502.
Geworski L, Knoop BO, de Cabrejas ML, Knapp WH, Munz DL. Recovery correction for quantitation in emission tomography: a feasibility study. Eur J Nucl Med 2000;27(2):161–9.
Soret M, Koulibaly PM, Darcourt J, Hapdey S, Buvat I. Quantitative accuracy of dopaminergic neurotransmission imaging with (123)I SPECT. J Nucl Med 2003;44(7):1184–93.
Soret M, Koulibaly PM, Darcourt J, Buvat I. Partial volume effect correction in SPECT for striatal uptake measurements in patients with neurodegenerative diseases: impact upon patient classification. Eur J Nucl Med Mol Imaging 2006;33(9):1062–72.
Hutton BF, Lau YH. Application of distance-dependent resolution compensation and post-reconstruction filtering for myocardial SPECT. Phys Med Biol 1998;43(6):1679–93.
Teo B, Seo Y, Bacharach SL, Carrasquillo JA, Libutti SK, Shukla H, et al. Partial-volume correction in PET: validation of an iterative postreconstruction method with phantom and patient data. J Nucl Med 2007;48(5):802–10.
Acknowledgement
The study centres wish to thank the Executive Committee of the EANM for establishing the EANM Research Ltd. (EARL) as an administrative framework for this pilot project of a European investigator initiated multi-centre trial. We thank ABX-CRO for managing the network activities. We appreciate the financial support of the ENCDAT project by GE Healthcare and the German Parkinson Association. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
An editorial commentary on this article can be found at DOI 10.1007/s00259-011-1996-5.
Rights and permissions
About this article
Cite this article
Dickson, J.C., Tossici-Bolt, L., Sera, T. et al. Proposal for the standardisation of multi-centre trials in nuclear medicine imaging: prerequisites for a European 123I-FP-CIT SPECT database. Eur J Nucl Med Mol Imaging 39, 188–197 (2012). https://doi.org/10.1007/s00259-011-1884-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1884-z