Abstract
Purpose
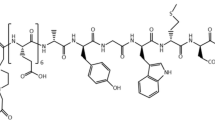
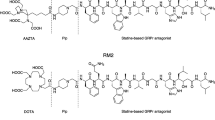
Specific overexpression of cholecystokinin 2 (CCK2)/gastrin receptors has been demonstrated in several tumours of neuroendocrine origin. In some of these cancer types, such as medullary thyroid cancer (MTC), a sensitive diagnostic modality is still unavailable and therapeutic options for inoperable lesions are needed. Peptide receptor radionuclide therapy (PRRT) may be a viable therapeutic strategy in the management of these patients. Several CCK2R-targeted radiopharmaceuticals have been described in recent years. As part of the European Union COST Action BM0607 we studied the in vitro and in vivo characteristics of 12 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-conjugated CCK2R binding peptides. In the present study, we analysed binding and internalization characteristics. Stability, biodistribution and imaging studies have been performed in parallel by other centres involved in the project.
Methods
Determination of IC50 values was performed using autoradiography, with DOTA-peptides displacing 125I-CCK from receptors on tissue sections from human tumours. Saturation binding and internalization experiments were performed using 111In-labelled peptides. The rat AR42J cell line and the human A431-CCK2R transfected cell line were utilized for in vitro experiments; dissociation constants (Kd) and apparent number of binding sites (Bmax) were determined. Internalization was determined in receptor-expressing cells by incubating with tracer amounts of peptide at 37 and 4°C for different times up to 120 min. Surface-bound peptide was then stripped either by acid wash or subsequent incubation with 1 μM unlabelled peptide at 4°C.
Results
All peptides showed high receptor affinity with IC50 values ranging from 0.2 to 3.4 nM. Saturation experiments also showed high affinity with Kd values in the 10−9–10−8 M range. Bmax values estimated in A431-CCK2R cells ranged from 0.6 to 2.2 × 106 per cell. All peptides showed high levels of internalization when incubated at 37°C.
Conclusion
All DOTA-conjugated peptides showed high receptor binding and internalization properties and appear suitable for further characterization, as described in other articles of this issue.


Similar content being viewed by others
References
Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 2003;24:389–427.
Reubi JC, Schaer JC, Waser B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res 1997;57:1377–86.
Roman S, Mehta P, Sosa JA. Medullary thyroid cancer: early detection and novel treatments. Curr Opin Oncol 2009;21:5–10.
Béhé M, Becker W, Gotthardt M, Angerstein C, Behr TM. Improved kinetic stability of DTPA-dGlu as compared with conventional monofunctional DTPA in chelating indium and yttrium: preclinical and initial clinical evaluation of radiometal labelled minigastrin derivatives. Eur J Nucl Med Mol Imaging 2003;30:1140–6.
Behr TM, Béhé M, Becker W. Diagnostic applications of radiolabeled peptides in nuclear endocrinology. Q J Nucl Med 1999;43:268–80.
Good S, Walter MA, Waser B, Wang X, Müller-Brand J, Béhé MP, et al. Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. Eur J Nucl Med Mol Imaging 2008;35:1868–77. doi:10.1007/s00259-008-0803-4.
Aloj L, Caracò C, Panico M, Zannetti A, Del Vecchio S, Tesauro D, et al. In vitro and in vivo evaluation of 111In-DTPAGlu-G-CCK8 for cholecystokinin-B receptor imaging. J Nucl Med 2004;45:485–94.
Reubi JC, Waser B, Schaer JC, Laederach U, Erion J, Srinivasan A, et al. Unsulfated DTPA- and DOTA-CCK analogs as specific high-affinity ligands for CCK-B receptor-expressing human and rat tissues in vitro and in vivo. Eur J Nucl Med 1998;25:481–90.
von Guggenberg E, Sallegger W, Helbok A, Ocak M, King R, Mather SJ, et al. Cyclic minigastrin analogues for gastrin receptor scintigraphy with technetium-99m: preclinical evaluation. J Med Chem 2009;52:4786–93. doi:10.1021/jm900400w.
Roosenburg S, Laverman P, Joosten L, Eek A, Oyen WJ, de Jong M, et al. Stabilized (111)In-labeled sCCK8 analogues for targeting CCK2-receptor positive tumors: synthesis and evaluation. Bioconjug Chem 2010;21:663–70. doi:10.1021/bc900465y.
Mather SJ, McKenzie AJ, Sosabowski JK, Morris TM, Ellison D, Watson SA. Selection of radiolabeled gastrin analogs for peptide receptor-targeted radionuclide therapy. J Nucl Med 2007;48:615–22.
Sosabowski JK, Matzow T, Foster JM, Finucane C, Ellison D, Watson SA, et al. Targeting of CCK-2 receptor-expressing tumors using a radiolabeled divalent gastrin peptide. J Nucl Med 2009;50:2082–9. doi:10.2967/jnumed.109.064808.
Nock BA, Maina T, Béhé M, Nikolopoulou A, Gotthardt M, Schmitt JS, et al. CCK-2/gastrin receptor-targeted tumor imaging with (99m)Tc-labeled minigastrin analogs. J Nucl Med 2005;46:1727–36.
Fröberg AC, de Jong M, Nock BA, Breeman WA, Erion JL, Maina T, et al. Comparison of three radiolabelled peptide analogues for CCK-2 receptor scintigraphy in medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging 2009;36:1265–72. doi:10.1007/s00259-009-1098-9.
Béhé M, Behr TM. Cholecystokinin-B (CCK-B)/gastrin receptor targeting peptides for staging and therapy of medullary thyroid cancer and other CCK-B receptor expressing malignancies. Biopolymers 2002;66:399–418. doi:10.1002/bip.10356.
Béhé M, Kluge G, Becker W, Gotthardt M, Behr TM. Use of polyglutamic acids to reduce uptake of radiometal-labeled minigastrin in the kidneys. J Nucl Med 2005;46:1012–5.
Laverman P, Roosenburg S, Gotthardt M, Park J, Oyen WJ, de Jong M, et al. Targeting of a CCK(2) receptor splice variant with (111)In-labelled cholecystokinin-8 (CCK8) and (111)In-labelled minigastrin. Eur J Nucl Med Mol Imaging 2008;35:386–92. doi:10.1007/s00259-007-0604-1.
von Guggenberg E, Dietrich H, Skvortsova I, Gabriel M, Virgolini IJ, Decristoforo C. 99mTc-labelled HYNIC-minigastrin with reduced kidney uptake for targeting of CCK-2 receptor-positive tumours. Eur J Nucl Med Mol Imaging 2007;34:1209–18. doi:10.1007/s00259-006-0348-3.
Ocak M, Helbok A, Rangger C, Peitl PK, Nock B, Morelli G, et al. Comparison of biological stability and metabolism of CCK2-receptor targeting peptides, a collaborative project under COST BM0607. Eur J Nucl Med Mol Imaging 2011, in press.
Laverman P, Joosten L, Eek A, Roosenburg S, Peitl PK, Maina T, et al. Comparative biodistribution of twelve gastrin/CCK2 receptor targeting peptides. Eur J Nucl Med Mol Imaging 2011, in press.
Sosabowski JK, Finucane C, Foster JM, Ellison D, Burnet J, Laverman P, et al. Comparison of 111In-labelled CCK2-receptor targeting peptides using NanoSPECT/CT imaging. Submitted for publication 2011.
Giragossian C, Mierke DF. Intermolecular interactions between cholecystokinin-8 and the third extracellular loop of the cholecystokinin-2 receptor. Biochemistry 2002;41:4560–6.
Roosenburg S, Laverman P, van Delft FL, Boerman OC. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino Acids 2010. doi:10.1007/s00726-010-0501-y.
D’Andrea LD, Testa I, Panico M, Di Stasi R, Caracò C, Tarallo L, et al. In vivo and in vitro characterization of CCK8 bearing a histidine-based chelator labeled with 99mTc-tricarbonyl. Biopolymers 2008;90:707–12. doi:10.1002/bip.21041.
Tornesello AL, Aurilio M, Accardo A, Tarallo L, Barbieri A, Arra C, et al. Gastrin and cholecystokinin peptide-based radiopharmaceuticals: an in vivo and in vitro comparison. J Pept Sci 2011;17:405–12. doi:10.1002/psc.1327.
Marsouvanidis PJ, Tatsi A, Nock BA, Krenning EP, Maina T, de Jong M. [111In]Sargastrin, a Gastrin I-based radioligand targeting CCK2-R-positive tumors in vivo. Eur J Nucl Med Mol Imaging 2009;36:S259.
Kolenc-Peitl P, Mansi R, Tamma ML, Gmeiner-Stopar T, Sollner-Dolenc M, Waser B, et al. Highly improved metabolic stability and pharmacokinetics of indium-111-DOTA-gastrin conjugates for targeting of the gastrin receptor. J Med Chem. In press 2011.
Acknowledgements
This work has been performed under COST Action BM0607 “Targeted Radionuclide Therapy”.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aloj, L., Aurilio, M., Rinaldi, V. et al. Comparison of the binding and internalization properties of 12 DOTA-coupled and 111In-labelled CCK2/gastrin receptor binding peptides: a collaborative project under COST Action BM0607. Eur J Nucl Med Mol Imaging 38, 1417–1425 (2011). https://doi.org/10.1007/s00259-011-1816-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1816-y