Abstract
Purpose
By targeting somatostatin receptors (sst) radiopeptides have been established for both diagnosis and therapy. For physiologically normal human tissues the study provides a normative database of maximum standardized uptake value (SUVmax) and sst mRNA.
Methods
A total of 120 patients were subjected to diagnostic 68Ga-DOTATOC positron emission tomography (PET)/CT (age range 19–83 years). SUVmax values were measured in physiologically normal tissues defined by normal morphology, absence of surgical intervention and absence of metastatic spread during clinical follow-up. Expression of sst subtypes (sst1–sst5) was measured independently in pooled adult normal human tissue by real-time reverse transcriptase polymerase chain reaction (RT-PCR).
Results
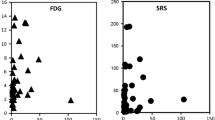
SUVmax revealed a region-specific pattern (e.g., mean ± SD, spleen 31.1 ± 10.9, kidney 16.9 ± 5.3, liver 12.8 ± 3.6, stomach 7.0 ± 3.1, head of pancreas 6.2 ± 2.3, small bowel 4.8 ± 1.8, thyroid 4.7 ± 2.2, bone 3.9 ± 1.3, large bowel 2.9 ± 0.8, muscle 2.1 ± 0.5, parotid gland 1.9 ± 0.6, axillary lymph node 0.8 ± 0.3 and lung 0.7 ± 0.3). SUVmax was age independent. Gender differences were evident within the thyroid (female/male: 3.7 ± 1.6/5.5 ± 2.4, p < 0.001; Mann-Whitney U test) and the pancreatic head (5.5 ± 1.9/6.9 ± 2.2, p < 0.001). The sst mRNA was widely expressed and heterogeneous, showing sst1 to be most abundant. SUVmax values exclusively correlated with sst2 expression (r = 0.846, p < 0.001; Spearman rank correlation analysis), whereas there was no correlation of SUVmax with the expression of the other four subtypes.
Conclusion
In normal human tissues 68Ga-DOTATOC imaging has been related to the expression of sst2 at the level of mRNA. The novel normative database may improve diagnostics, monitoring and therapy of sst-expressing tumours or inflammation on a molecular basis.




Similar content being viewed by others
References
Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci 1995;16:86–8.
Lewin MJ. The somatostatin receptor in the GI tract. Annu Rev Physiol 1992;54:455–68.
Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999;20:157–98.
Ballian N, Brunicardi FC, Wang XP. Somatostatin and its receptors in the development of the endocrine pancreas. Pancreas 2006;33:1–12.
Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 2001;28:836–46.
Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol 2009;49:1–30.
Van Op den Bosch J, Adriaensen D, Van Nassauw L, Timmermans JP. The role(s) of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract: a review. Regul Pept 2009;156:1–8.
Patel YC, Srikant CB. Somatostatin receptors. Trends Endocrinol Metab 1997;8:398–405.
Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000;27:273–82.
Görges R, Kahaly G, Müller-Brand J, Mäcke H, Roser HW, Bockisch A. Radionuclide-labeled somatostatin analogues for diagnostic and therapeutic purposes in nonmedullary thyroid cancer. Thyroid 2001;11:647–59.
Gabriel M, Oberauer A, Dobrozemsky G, Decristoforo C, Putzer D, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med 2009;50:1427–34.
Rominger A, Saam T, Vogl E, Ubleis C, la Fougère C, Förster S, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med 2010;51:193–7.
van Hagen PM, Dalm VA, Staal F, Hofland LJ. The role of cortistatin in the human immune system. Mol Cell Endocrinol 2008;286:141–7.
Lincke T, Singer J, Kluge R, Sabri O, Paschke R. Relative quantification of indium-111 pentetreotide and gallium-68 DOTATOC uptake in the thyroid gland and association with thyroid pathologies. Thyroid 2009;19:381–9.
Dalm VA, van Hagen PM, van Koetsveld PM, Achilefu S, Houtsmuller AB, Pols DH, et al. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am J Physiol Endocrinol Metab 2003;285:E344–53.
Hofmann M, Maecke H, Börner R, Weckesser E, Schöffski P, Oei L, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med 2001;28:1751–7.
Ruf J, Heuck F, Schiefer J, Denecke T, Elgeti F, Pascher A, et al. Impact of multiphase 68Ga-DOTATOC-PET/CT on therapy management in patients with neuroendocrine tumors. Neuroendocrinology 2010;91:101–9.
Putzer D, Gabriel M, Henninger B, Kendler D, Uprimny C, Dobrozemsky G, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med 2009;50:1214–21.
Corleto VD, Falconi M, Panzuto F, Milione M, De Luca O, Perri P, et al. Somatostatin receptor subtypes 2 and 5 are associated with better survival in well-differentiated endocrine carcinomas. Neuroendocrinology 2009;89:223–30.
Barone R, Borson-Chazot F, Valkema R, Walrand S, Chauvin F, Gogou L, et al. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med 2005;46 Suppl 1:99S–106S.
Cremonesi M, Botta F, Di Dia A, Ferrari M, Bodei L, De Cicco C, et al. Dosimetry for treatment with radiolabelled somatostatin analogues. A review. Q J Nucl Med Mol Imaging 2010;54:37–51.
Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging 2010;54:61–7.
Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med 2007;48:1741–8.
Jentzen W. Experimental investigation of factors affecting the absolute recovery coefficients in iodine-124 PET lesion imaging. Phys Med Biol 2010;55:2365–98.
Ziegler CG, Brown JW, Schally AV, Erler A, Gebauer L, Treszl A, et al. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: antiproliferative effects of peptide analogues. Proc Natl Acad Sci U S A 2009;106:15879–84.
Ueberberg B, Tourne H, Redmann A, Walz MK, Schmid KW, Mann K, et al. Differential expression of the human somatostatin receptor subtypes sst1 to sst5 in various adrenal tumors and normal adrenal gland. Horm Metab Res 2005;37:722–8.
Ueberberg B, Unger N, Sheu SY, Walz MK, Schmid KW, Saeger W, et al. Differential expression of ghrelin and its receptor (GHS-R1a) in various adrenal tumors and normal adrenal gland. Horm Metab Res 2008;40:181–8.
Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, Santini D, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med 2010;51:353–9.
Koukouraki S, Strauss LG, Georgoulias V, Eisenhut M, Haberkorn U, Dimitrakopoulou-Strauss A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging 2006;33:1115–22.
Froidevaux S, Eberle AN, Christe M, Sumanovski L, Heppeler A, Schmitt JS, et al. Neuroendocrine tumor targeting: study of novel gallium-labeled somatostatin radiopeptides in a rat pancreatic tumor model. Int J Cancer 2002;98:930–7.
Kessler RM, Ellis Jr JR, Eden M. Analysis of emission tomographic scan data: limitations imposed by resolution and background. J Comput Assist Tomogr 1984;8:514–22.
Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 2001;28:836–46.
Zilles K, Palomero-Gallagher N, Grefkes C, Scheperjans F, Boy C, Amunts K, et al. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol 2002;12:587–99.
Ludvigsen E, Olsson R, Stridsberg M, Janson ET, Sandler S. Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. J Histochem Cytochem 2004;52:391–400.
Bhandari S, Watson N, Long E, Sharpe S, Zhong W, Xu SZ, et al. Expression of somatostatin and somatostatin receptor subtypes 1–5 in human normal and diseased kidney. J Histochem Cytochem 2008;56:733–43.
Low MJ. Clinical endocrinology and metabolism. The somatostatin neuroendocrine system: physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Pract Res Clin Endocrinol Metab 2004;18:607–22.
Bryant MG, Bloom SR, Polak JM, Hobbs S, Domschke W, Domschke S, et al. Measurement of gut hormonal peptides in biopsies from human stomach and proximal small intestine. Gut 1983;24:114–9.
Smith WH, Nair RU, Adamson D, Kearney MT, Ball SG, Balmforth AJ. Somatostatin receptor subtype expression in the human heart: differential expression by myocytes and fibroblasts. J Endocrinol 2005;187:379–86.
Eckelman WC. The application of receptor theory to receptor-binding and enzyme-binding oncologic radiopharmaceuticals. Nucl Med Biol 1994;21:759–69.
Meyer PT, Elmenhorst D, Boy C, Winz O, Matusch A, Zilles K, et al. Effect of aging on cerebral A1 adenosine receptors: a [18F]CPFPX PET study in humans. Neurobiol Aging 2007;28:1914–24.
Acknowledgements
We appreciate the skilful technical support of Julia Joos, Tylay Kilic, Kathrin Klemm, Dipl. Ing. Harald Lahmer, Janina Markese-Asgari, Sandra Schneider, Dr. Jochen Schmitz, Martha Senkowski, Dipl. Ing. Wilfried Sonnenschein, Dipl. Ing. Isabell Stergar, and cand. med. Can Yüksel.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table. S1
Sequence and localization of somatostatin receptor (sst) primers as well as annealing temperature (T) used for quantitative reverse transcriptase polymerase chain reaction (RT-PCR) data of regional somatostatin receptor expression and size of PCR products (DOC 52 kb)
Table. S2
Supplementary material: Tissue and RNA samples and quantitative real-time RT-PCR. Primers and probes for all target sequences (Table 1) were designed using Primer Express software (PE Applied Biosystems, Warrington, UK). The probes were labelled with a fluorescent dye (6-carboxyfluorescein) and a quencher dye (6-carboxytetramethylrhodamine) (MWG-Biotech, Ebersberg, Germany). PCR reactions were performed using the ABI 7300 Real Time PCR system (Applied Biosystems) and the One-Step RT-PCR Master Mix Reagents Kit from Applied Biosystems. The reactions contained 100 ng RNA sample or serial dilutions of sst-specific cDNAs, the selective receptor’s subtype probe (100 nM) and specific primers. The following experimental protocol was used: reverse transcription (48°C for 30 min), AmpliTaq Gold Activation (95°C for 10 min), PCR program of two temperature cycles repeated 40 times (95°C for 15 s and 60°C for 1 min). One no-template control was included as negative control in every amplification run. The human housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control, quantitated by parallel amplification in samples and control tissues (Control-Kit GAPDH, Applied Biosystems). Quantitation of mRNA samples was carried out by relating the PCR threshold cycles obtained from respective samples to cDNA plasmid-specific standard curves. Standard curves were received by plotting the log (calculated copy number) against the threshold cycle. The relative quantities (N) of unknown samples were calculated from the regression line according to the formula: log N = (CT – b)/m, where CT is the threshold cycle, b the y-intercept, and m the slope of the standard curve line. Sst expression levels are presented as the mRNA copy numbers per microgram of total RNA. Copy numbers were calculated as follows: molecules/ng = (1×10−9 /MW × N0 molecules/mole, where MW is the molecular weight of the specific sst receptor subtype and N0 = Avogadro’s number, 6.023 × 1023). Amplifications of respective samples were carried out in triplicate. A detection limit of 25 copies of specific RNA molecules/μg total RNA for all five sst subtypes was determined using the upper limit of cycles. Expression below that limit was considered as absent. To determine the intra-assay variability, the coefficient of variance (CV) was determined for one sample run 10 times in one experiment. CV values were calculated by standard deviation/mean × 100. The CV analysed was 12.92%. To determine the inter-assay variability, the CV was calculated for duplicate readings of one sample, which were run over seven separate occasions. The CV analysed was 16.09% (DOC 25 kb)
Rights and permissions
About this article
Cite this article
Boy, C., Heusner, T.A., Poeppel, T.D. et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1–sst5) expression in normal human tissue: correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging 38, 1224–1236 (2011). https://doi.org/10.1007/s00259-011-1760-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1760-x